3469
Selective probe of intra- and extra-cellular diffusion with non-invasive MRI using non-uniform oscillating gradients1Instituto Balseiro, CNEA, Universidad Nacional de Cuyo, S. C. de Bariloche, Argentina, 2Centro Atómico Bariloche, CONICET, CNEA, S. C. de Bariloche, Argentina, 3Instituto de Nanociencia y Nanotecnologia, CNEA, CONICET, S. C. de Bariloche, Argentina
Synopsis
Keywords: Microstructure, Diffusion/other diffusion imaging techniques
Motivation: Overcoming MRI resolution limitations is crucial for early-diagnosis of cellular-level pathologies. Achieving non-invasive MRI-images with cellular-resolution promises deeper insights into tissue-microstructure for advancing medical diagnosis.
Goal(s): We aim to obtain cellular-resolution by characterizing molecular diffusion within intra- and extra-cellular compartments. We use Non-uniform Oscillating-Gradient Spin-Echo (NOGSE) signal-filtering potential, to address this intricate molecular diffusion problem.
Approach: We employ diffusion-theory and the NOGSE-sequence, to quantitatively characterize yeast-cell phantoms mimicking tissue-microstructure.
Results: We successfully characterize distinct diffusion-regimes within and outside cells. We introduce an innovative method to discriminate intra- and extra-cellular signals without complex models and analyses, allowing selective measurements of specific tissue-microstructure compartments.
Impact: We introduce a transformative approach to non-invasive imaging, allowing cellular-level resolution discriminating different tissue-microstructure features without model assumptions. It offers a tool to investigate intricate cellular structures. Harnessing this technology might benefit patient early-diagnosis of diseases, based on quantitative images.
Introduction
Our study is motivated by the dual goals of advancing our understanding of cellular microstructure probed by molecular diffusion1-7 while harnessing the potential of using Non-uniform Oscillating-Gradient Spin-Echo (NOGSE) sequence8-10 for this purpose. These insights carry far-reaching implications, as understanding cellular microstructure and molecular diffusion in complex systems is vital for medical sciences, offering opportunities for enhanced diagnostics and therapeutic strategies11-14.In this context, our work represents a step forward to unlock the potential of NOGSE as a powerful tool for non-invasive cellular microstructure characterization, enabling the precise separation of signals originating from within and outside cells. The key advantage is that it facilitates precise investigations without the need for complex mathematical models and analyses. These results hold substantial promise as a diagnosis paradigm for the understanding of tissue microstructure to address critical questions connected with diseases11-14.
Methods
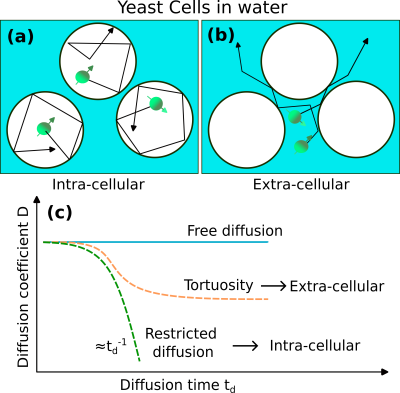
Tissue phantomWe imitate cellular tissue properties, using fresh saccharomyces cerevisiae commercial yeast cells dissolved in water (Fig. 1). We wait until cells are deposited at the bottom of a plastic tube. Previous work10 on similar samples have shown that the mean diameter of cells is around 5.8 $$$\mu$$$m. We assume the cells have a spherical geometry.
Diffusion weighted imaging
Diffusion weighted MRI experiments were performed in a 9.4T preclinical-scanner (Bruker Avance III HD WB-NMR). We exploit the signal decay due to dephasing induced by the molecular diffusion that contains information of the sample microstructure. Utilizing time-modulated magnetic field gradients enables the probing of the spins' Brownian motion, which is governed by the microstructural morphology of the sample, thereby facilitating its characterization8-10,15-17.
Pulsed-Gradient Spin-Echo probing
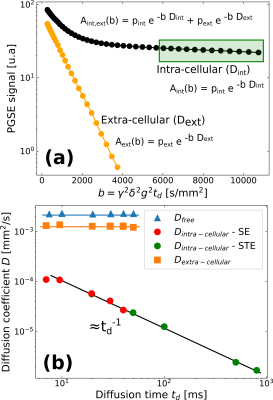
We conducted Pulsed-Gradient Spin-Echo (PGSE) experiments on the yeast cells18-19, with diffusion times systematically varied (Fig. 1b). We quantified the apparent diffusion coefficients for each diffusion time. We performed bi-exponential fittings to discern and extract the diffusion coefficients specific to the extra- and intra-cellular spaces (Fig. 2).
Selective diffusion probing by Non-uniform Oscillating Gradient Spin-echo
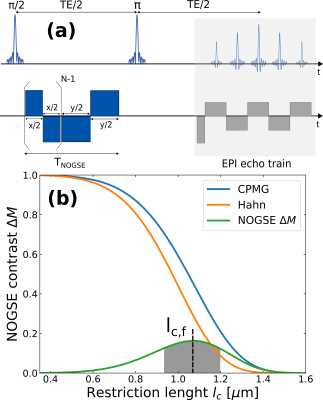
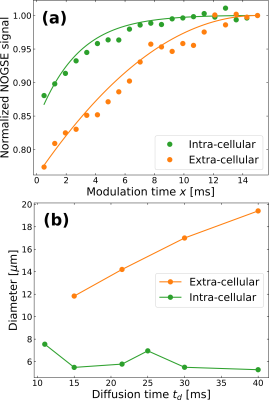
We employed the Non-uniform Oscillating Gradient Spin-Echo (NOGSE) technique8-10 to selectively measure diffusion processes in our sample (Fig. 3a). NOGSE allowed us to filter signals from spins diffusing within specific restriction-sizes20 (Fig. 3b). By adjusting control parameters, we scan and measure these restriction-sizes based on the signal evolution over different diffusion times (Fig. 4).
Results and Discussions
Intra- and extra-cellular apparent diffusion coefficientsWe identified two diffusion regimes within the yeast cells phantom (Fig. 2). We extracted the apparent diffusion coefficients by fitting a bi-exponential model to the experimental data with varying b-values. The extra-cellular diffusion, characterized by a constant diffusion coefficient smaller than free water diffusion, suggests tortuous diffusion. The intracellular component exhibits a diffusion coefficient inversely proportional to the diffusion-time, indicating restricted diffusion.
NOGSE size-filters of intra- and extra-cellular compartments
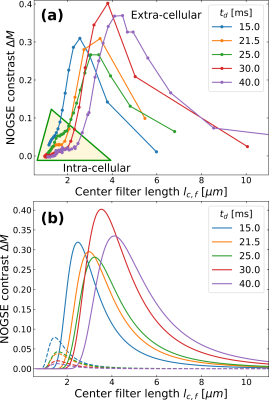
Using NOGSE, we achieved selective separation of signals from within and outside the yeast cells, eliminating the need for complex diffusion models (Fig. 4). This means that we observed a bimodal distribution based on the NOGSE signal filter, where one mode is static (intracellular) and the other exhibits a time-dependent increase (extracellular) as a function of the diffusion-time (Fig. 4b). This dynamic behavior in the extracellular component is consistent with previous observations of extra-axonal (extra-cellular) phantom-models21.
Selective estimation of intra- and extra-cellular restriction-sizes
NOGSE's selective signal filtration showcases its ability to determine cellular sizes while filtering out extra-cellular signals (Fig. 5). The mean cell diameter estimation is in agreement with measurements obtained through optical microscopy10, underscoring NOGSE's reliability and its capacity to provide comprehensive data on cellular microstructure. Moreover, it can also determine selectively, extra-cellular microstructure sizes determined by a microtortuosity phenomenon evidencing an effective restriction size that grows as a function of the diffusion time, consistently with previous observations with extra-cellular (extra-axonal) phantom models21.
Conclusions
Our study highlights the capability of NOGSE to selectively filter two distinct water diffusion regimes, enabling precise investigations of specific microstructure regions without the need for complex mathematical models and analysis. Specifically, it offers an approach to selectively target and investigate distinct tissue microstructure regions, allowing a focused analysis of intra- and extra-cellular compartments. This feature offers versatility across a wide-range of parameters, making NOGSE advantageous over standard PGSE. Additionally, NOGSE requires lower diffusion-times and gradient strengths for cell diameter determination. Our experiments provided insights into the short-time diffusion processes within the extracellular compartments evidencing effective restriction-sizes that grow as a function of time, which remains an area for future investigation. Overall, NOGSE shows promise as a non-invasive tool for cellular microstructure studies, contributing to our understanding of cellular processes that might be connected to diseases.Acknowledgements
This work was supported by CNEA; CONICET; ANPCyT-FONCyT PICT-2017-3156, PICT-2017-3699, PICT-2018-4333, PICT-2021-GRF-TI-00134, PICT-2021-I-A-00070; PIP-CONICET (11220170100486CO); UNCUYO SIIP Tipo I 2019-C028, 2022-C002, 2022-C030; Instituto Balseiro; Collaboration programs between the MINCyT (Argentina) and, MAECI (Italy) and MOST (Israel); and Erasmus+ Higher Education program from the European Commission between the CIMEC (University of Trento) and the Instituto Balseiro (Universidad Nacional de Cuyo).References
1. Gore JC, Xu J, Colvin DC, Yankeelov TE, Parsons EC, Does MD. Characterization of tissue structure at varying length scales using temporal diffusion spectroscopy. NMR Biomed. 2010; 23:745.
2. White NS, et al. Probing tissue microstructure with restriction spectrum imaging: Histological and theoretical validation. Hum. Brain Mapp. 2013; 34:327.
3. Nilsson M, Englund E, Szczepankiewicz F, van Westen D, Sundgren PC. Imaging brain tumour microstructure. Neuroimage. 2018; 182:232-250.
4. Xu J, Jiang X, Li H, Arlinghaus LR, McKinley ET, Devan SP, Hardy BM, Xie J, Kang H, Chakravarthy AB, Gore JC. Magnetic resonance imaging of mean cell size in human breast tumors. Magn. Reson. Med. 2020; 83:2002.
5. Zwick A, Suter D, Kurizki G, Álvarez GA. Precision limits of tissue microstructure characterization by Magnetic Resonance Imaging. Phys. Rev. Applied 2020; 14;024088.
6. Yon M, de Almeida Martins JP, Bao Q, Budde MD, Frydman L, Topgaard D. Diffusion tensor distribution imaging of an in vivo mouse brain at ultrahigh magnetic field by spatiotemporal encoding. NMR Biomed. 2020; 33:e4355.
7. Zwick A, Álvarez GA. Quantum sensing tools to characterize physical, chemical and biological processes with magnetic resonance. J. Magn. Reson. Open 2023; 16-17:100113.
8. Álvarez GA, Shemesh N, Frydman L. Coherent dynamical recoupling of diffusion-driven decoherence in magnetic resonance. Phys. Rev. Lett. 2013; 111:080404.
9. Shemesh N, Álvarez GA, Frydman L. Measuring small compartment dimensions by probing diffusion dynamics via Non-uniform Oscillating-Gradient Spin-Echo (NOGSE) NMR. J. Magn. Reson. 2013; 237:49.
10. Shemesh N, Álvarez GA, Frydman L. Size distribution imaging by Non-Uniform Oscillating-Gradient Spin Echo (NOGSE) MRI. PLoS ONE 2015; 10:e0133201.
11. Padhani AR, et al. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: Consensus and recommendations. Neoplasia 2009; 11:102.
12. Drago V., et al. Disease tracking markers for alzheimer’s Disease at the prodromal (MCI) stage. J. Alzheimers Dis. 2011; 26:159.
13. White NS, et al. Diffusion-weighted imaging in cancer: Physical foundations and applications of restriction spectrum imaging. Cancer Res. 2014; 74:4638.
14. Huang SY, Fan Q, Machado N, et al. Corpus callosum axon diameter relates to cognitive impairment in multiple sclerosis. Ann. Clin. Transl. Neurol. 2019; 6(5):882-892.
15. Stepišnik J. Time-dependent self-diffusion by NMR spin-echo. Physica B 1993; 183: 343–350.
16. Callaghan PT, Stepisnik J. Frequency-domain analysis of spin motion using modulated-gradient NMR. J. Magn. Reson. A 1995; 117:118-122.
17. Stepisnik J, et al. Spectral characterization of diffusion in porous media by the modulated gradient spin echo with CPMG sequence. Journal of Magnetic Resonance. 2006; 182:195.
18. Stejskal EO, Tanner JE. Spin diffusion measurements – spin echoes in presence of a time-dependent field gradient. J. Chem. Phys. 1965; 42:288–292.
19. Suh K-J, et al. Water self-diffusion behavior in yeast cells studied by pulsed field gradient NMR. Biophysical Chemistry. 2003; 104:121.
20. Capiglioni M, Zwick A, Jiménez P, Álvarez GA. Noninvasive Quantitative Imaging of Selective Microstructure Sizes via Magnetic Resonance. Phys. Rev. Applied 2021; 15:014045.
21. Saidman EL, Zwick A, Tambalo S, Feiweier T, Jovicich J, and Álvarez GA. Selective filters of translational molecular diffusion dynamics in white matter microstructures with preclinical and clinical MRI. Proc. Intl. Soc. Mag. Reson. Med. 2023; 31:0970.
Figures