0116
Selective filters of translational molecular diffusion dynamics in human brain microstructures1Centro Atómico Bariloche, CONICET, CNEA, Bariloche, Argentina, Bariloche, Argentina, 2Instituto de Nanociencia y Nanotecnologia, CNEA, CONICET, Bariloche, Argentina, Bariloche, Argentina, 3Instituto Balseiro, CNEA, Bariloche, Argentina, Bariloche, Argentina, 4Center for Mind/Brain Sciences - CIMeC, University of Trento, Rovereto, Italy, Rovereto, Italy, 5Siemens Healthcare GmbH, Erlangen, Germany, Erlangen, Germany
Synopsis
Keywords: Microstructure, Diffusion/other diffusion imaging techniques
Motivation: Our primary aim is to enhance non-invasive tissue-microstructure characterization as a diagnostic paradigm through advanced MRI methods.
Goal(s): We explore microscopic tortuosity in human white-matter using the Non-uniform Oscillating-Gradient Spin-Echo (NOGSE) contrast in a clinical 3T MRI-scanner.
Approach: The NOGSE contrast was obtained by subtracting distinct OGSE acquisitions, allowing the discrimination of signals from molecules within specific brain compartments.
Results: We found restriction-sizes consistent with human histological findings, and evidence that the dominant signals originate from extra-axonal spaces, supporting microscopic tortuosity effects. This compartment-size specific contrast opens a path for diagnosis based on quantitative imaging.
Impact: We characterize microscopic-tortuosity mechanisms in human-brain white-matter through the Non-uniform Oscillating-Gradient Spin-Echo contrast, which targets the signal of confined molecules in specific microscopic-sizes. This novel contrast, demonstrated at 3T-MRI, promises a quantitative tissue-microstructure paradigm for medical diagnosis of diseases.
Introduction
Magnetic resonance imaging (MRI) is a vital non-invasive tool for medical imaging with unveiled potential for clinical diagnosis. It holds the promise of non-invasively extracting quantitative information characterizing tissue features on microscopic scales by observing water molecule diffusion processes1,2. These measures can be of clinical relevance, for example, for early diagnosis of cancer and neurodegenerative diseases3-7. However, biological tissues are very complex, exhibiting intricate molecular diffusion patterns, thus imposing huge challenges in obtaining specific and reliable spatial information. To approach these outstanding problems, we measure for the first time Non-uniform Oscillating Gradient Spin Echo (NOGSE) image contrasts2,8 in healthy human brains to observe diffusion processes on selective compartments of specific microstructure-sizes9.Our study reveals a novel microscopic tortuosity mechanism within the human corpus callosum white matter fibers. We found that the dominant contrast generated on diffusion weighted images has a strong component of extra-axonal signal, and that the inferred restriction-sizes probably comes from extra-axonal compartments10. Our approach thus enables the extraction of microstructural insights from white-matter axonal-tracts, that can shed light on interpreting the complex molecular diffusion within intra- and extra-axonal compartments to extract more reliable quantitative information.
Methods
Theory: Probing molecular diffusion in intra- and extra-axonal compartmentsThe NOGSE-contrast1-2,8 selectively highlights the signal from molecules confined within specific microstructure-sizes9-10. To characterize the hindered diffusion in the frequency domain probed by NOGSE1-2,8, we use the phenomenological model for the spectral density10-12.
$$S(\omega)=\frac{D_0}{\alpha\omega^2}+(1-\frac{1}{\alpha})\frac{D_0\tau_c^2}{1+\omega^2\tau_c^2} \;\;\; (1)$$
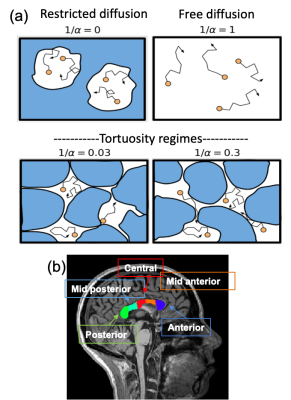
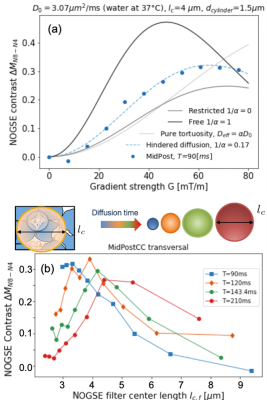
Here $$$D_0$$$ is the free-diffusion coefficient, $$$\tau_c$$$ the characteristic-time for exploring compartment sizes $$$l_c=\sqrt{D_0\tau_c}$$$, and $$$\alpha$$$ the tortuosity coefficient. Long diffusion times (T) lead to the tortuosity diffusion coefficient $$$D_0/\alpha$$$, and fully restricted diffusion occurs when $$$1/\alpha→0$$$. We model intra- and extra-axonal space diffusion based on this assumption (Fig. 1(a)).
Clinical MR acquisition on healthy volunteers
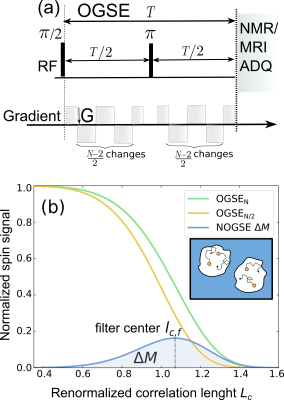
A 3T clinical scanner (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany) was used to scan three healthy volunteers who gave informed consent. Following our phantom validation study10, a PGSE sequence was used to first characterize the diffusion process and determine the tortuosity coefficient $$$\alpha$$$ (Fig. 1(a)). The NOGSE-contrast to selectively observe diffusion processes9, was determined by subtracting data from different gradient modulation cycles of an OGSE research application10 (Fig. 2). A standard T1-weighted anatomical image was used for brain tissue segmentation.
Corpus callosum
We focused on the corpus callosum (CC), which was automatically segmented from the T1 MRI using Freesurfer, separating its anterior, mid-anterior, central, mid-posterior and posterior portions (Fig. 1(b)). The CC is a good model to evaluate tortuosity because it contains aligned axonal-tracts containing intra- and extra-axonal compartments where water molecules diffuse, with different diameters across its compartments as shown by histology13. After co-registering with diffusion data, the CC regions were used as masks to average the NOGSE contrast.
Results and Discussions
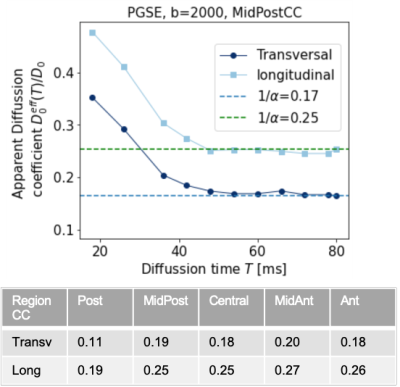
PGSE sequences14 at long diffusion times determine tortuosity coefficient $$$\alpha$$$ in the CC of healthy human brains (see Fig. 3).NOGSE microstructure-size filters9 in human brain
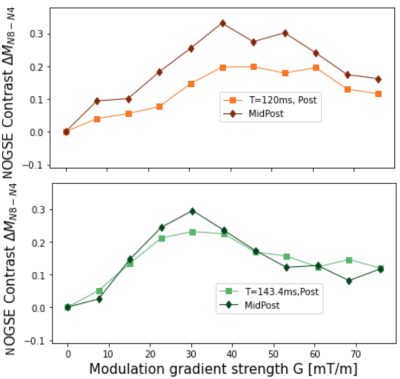
We measured the NOGSE-contrast, subtracting two OGSE-signals10. Figure 4 shows higher NOGSE-contrast for the MidPosterior, compared to the Posterior region of the CC, for low gradients, and reveals a trend of inverting the contrast relation for larger gradients. This is consistent with lower microstructure sizes, such as axon diameters, in posterior CC regions .
Consistency of model representations for the experimental data
We fit the CC experimental data based on the representation of Eq. (1) for different gradient directions. Only a model accounting for hindered diffusion describes the experimental data (see Fig. 5(a)).
Manifestation of microscopic tortuosis
The estimated effective restriction size increases with diffusion time, as seen in Fig. 5(b) for NOGSE-contrast as a function of the filtered restriction-size. This is consistent with a microscopic tortuosity mechanism observed in previous studies on extra-axonal diffusion phantoms10. Notably, water molecules exhibit restricted diffusion with an effective restriction size that expands over the probed diffusion-time.
Conclusions
With a clinical 3T MRI, we measured for the first time NOGSE-contrasts in healthy human corpus callosum, subtracting signals of varying OGSE-frequencies. This isolates signals from diffusing molecules confined to specific restriction sizes, thus characterizing more specific tissue microstructure compartments. Compartment sizes align with prior observations and histological data15-16. We found strong evidence that dominant signals emerge from extra-axonal spaces, consistent with microscopic tortuosity effects where restriction sizes grow with diffusion-time10. While further analysis and theoretical developments are needed, these results show the potential for selectively and quantitatively discerning signals from the convoluted intra- and extra-axonal spaces in the human brain, potentially advancing non-invasive MRI diagnostics.Acknowledgements
This work was supported by CNEA; CONICET; ANPCyT-FONCyT PICT-2017-3156, PICT-2017-3699, PICT-2018-4333, PICT-2021-GRF-TI-00134, PICT-2021-I-A-00070; PIBAA 2022-2023 28720210100635CO, PIP-CONICET (11220170100486CO); UNCUYO SIIP Tipo I 2019-C028, 2022-C002, 2022-C030; Instituto Balseiro; Collaboration programs between the MINCyT (Argentina) and, MAECI (Italy) and MOST (Israel); and Erasmus+ Higher Education program from the European Commission between the CIMEC (University of Trento) and the Instituto Balseiro (Universidad Nacional de Cuyo). Thorsten Feiweier is an employee of Siemens Healthcare GmbH, owns stocks of Siemens (Healthineers) AG and holds patents filed by Siemens.References
1. Álvarez GA, Shemesh N, Frydman L. Coherent dynamical recoupling of diffusion-driven decoherence in magnetic resonance. Phys. Rev. Lett. 2013; 111:080404.2. Shemesh N, Álvarez GA, Frydman L. Measuring small compartment dimensions by probing diffusion dynamics via Non-uniform Oscillating-Gradient Spin-Echo (NOGSE) NMR. J. Magn. Reson. 2013; 237:49.
3. Padhani AR, et al.,Diffusion-weighted magnetic resonance imaging as a cancer biomarker: Consensus and recommendations, Neoplasia 2009; 11:102.
4. Drago V., et al. Disease tracking markers for alzheimer’s Disease at the prodromal (MCI) stage. J. Alzheimers Dis. 2011); 26:159 .
5. White NS, et al. Probing tissue microstructure with restriction spectrum imaging: Histological and theoretical validation. Hum. Brain Mapp. 2013; 34:327.
6. White NS, et al. Diffusion-weighted imaging in cancer: Physical foundations and applications of restriction spectrum imaging, Cancer Res. 2014; 74:4638.
7. Alexander DC, Dyrby TB, Nilsson M, Zhang H. Imaging brain microstructure with diffusion mri: Practicality and applications. NMR Biomed. 2019; 32:e3841.
8. Shemesh N, Álvarez GA, Frydman L. Size distribution imaging by Non-Uniform Oscillating-Gradient Spin Echo (NOGSE) MRI. PLoS ONE 2015; 10:e0133201.
9. Capiglioni M, Zwick A, Jiménez P, Álvarez GA. Noninvasive Quantitative Imaging of Selective Microstructure Sizes via Magnetic Resonance. Phys. Rev. Applied 2021; 15:014045.
10. Saidman E., Zwick A., Tambalo S. , Feiweire, T., Jovicich J., Álvarez G. A. Selective filters of translational molecular diffusion dynamics in white matter microstructures with preclinical and clinical MRI Proc. Intl. Soc. Mag. Reson. Med. 2023; 31:3157.
11. Stepišnik J. Time-dependent self-diffusion by NMR spin-echo. Physica B 1993; 183: 343–350.
12. Stepišnik J, Mohorič A., Duh A. Diffusion and flow in a porous structure by the gradient spin echo spectral analysis. Physica B 2001; 307:158–168.
13. Caminiti R., Ghaziri H., Galuske R., Hof P. R., Innocenti G. M. Evolution amplified processing with temporally dispersed slow neuronal connectivity in primates. PNAS 2009; 106(46):19551.
14. Stejskal EO, Tanner JE. Spin diffusion measurements – spin echoes in presence of a time-dependent field gradient. J. Chem. Phys. 1965; 42:288–292.
15. Veraart, J., Nunes, D., Rudrapatna, U., Fieremans, E., Jones, D.K., Novikov, D.S. and Shemesh, N. Noninvasive quantification of axon radii using diffusion MRI. elife 2020; 9:49855.
16. Huang, S.Y., Tian, Q., Fan, Q. et al. High-gradient diffusion MRI reveals distinct estimates of axon diameter index within different white matter tracts in the in vivo human brain. Brain Struct Funct 2020; 225:1277
Figures