4492
Optimization of non-uniform oscillating gradient spin echo sequences for selective microstructure-size imaging1Instituto Balseiro, CNEA, Universidad Nacional de Cuyo, Bariloche, Argentina, 2Centro Atómico Bariloche, CONICET, CNEA, Bariloche, Argentina, 3Support Center for Advanced Neuroimaging (SCAN), University of Bern, Bern, Switzerland, 4Instituto de Nanociencia y Nanotecnologia, CONICET, CNEA, Bariloche, Argentina
Synopsis
Keywords: Diffusion/other diffusion imaging techniques, New Signal Preparation Schemes, NOGSE, Axon Diameter
Diffusion-weighting imaging is a promising method for quantitative imaging of tissue microstructure features that may identify early stages of pathologies. Non-uniform oscillating gradient spin-echo (NOGSE) sequences are a novel technique to study diffusion spectra and quantitatively characterize tissue. The sequence contrasts the signal between two modulating gradient frequencies to filter the signal originating from molecules diffusion in specific restriction sizes. We determine the range of control parameters that maximizes NOGSE contrast for filtering the restriction sizes. We assess the restriction sizes that this sequence can filter with current technologies.Introduction
An essential building block for identifying early stages of pathologies and their mechanism is tissue-microstructure characterization. However, it is currently difficult to observe non-invasively trustworthy biomarkers based on microstructure details1-2. A promising method for quantitatively estimating morphological characteristics of tissue microstructure is diffusion-weighting imaging (DWI)3-10. Recently, we found that a DWI technique based on the Non-uniform Oscillating Gradient Spin Echo sequence (NOGSE)7-9, filters information originating from molecules whose diffusion is restricted in specific sizes9. In this work, we determine the control sequence parameters that maximize the NOGSE contrast that selectively filters the microstructure sizes.Methods
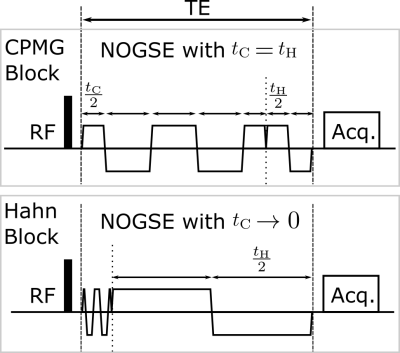
NOGSE sequence concatenates CPMG and Hahn gradient modulations (Fig. 1). The NOGSE contrast $$$\Delta M_{NOGSE}$$$ is the difference between two acquisitions with different gradient modulating frequencies at the limits $$$t_\mathrm{H} = t_\mathrm{C}$$$ (CPMG) and $$$t_\mathrm{C} \rightarrow 0$$$ (Hahn). $$$\Delta M_{NOGSE}$$$ probes the diffusion spectrum via a decay-shift between both acquisitions, while factoring out the influence of distorting effects7-9. We use the analytical expression of $$$\Delta M_{NOGSE}$$$7 to identify the regimes that evidence restricted-diffusion9$$\Delta M_{NOGSE}^{restr} \approx e^{-\gamma^{2}G^{2}D_{0}\tau_{c}^{3}(\frac{T_{E}}{\tau_{c}}-3)}(e^{\gamma^{2}G^{2}D_{0}\tau_{c}^{3}2(N-1)}-1)$$
with $$$\gamma$$$, $$$G$$$, and $$$D_0$$$ the gyromagnetic ratio, gradient strength, and free diffusion coefficient respectively. The correlation time of the molecular diffusion is $$$\tau_c=l_c^2/D_0$$$ where the restriction length $$$l_c$$$ is related to a microstructural morphologic parameter that restricts the diffusion. For example, approximating axons by a cylinder, their diameter is $$$d \approx 2.7 l_c$$$11. Then, $$$\Delta M_{NOGSE}$$$ acts as a size-microstructure filter since its amplitude directly reflects the restriction size. We thus calculate $$$\Delta M_{NOGSE}$$$ as a function of the renormalized decay constant $$$\gamma^2 G^2 D_0 \tau_c^3$$$ and the renormalized diffusion time $$$\frac{TE}{\tau_cN}$$$ to define the optimal range where restricted diffusion effects are reached. Within this regime, $$$\Delta M^r_{NOGSE}$$$ acts as a size-microstructure filter, thus we determine its filter center as an effective restriction diffusion length $$$L_c=(\tau_c^{2/3}\gamma^{2/3}G^{2/3}D_0^{1/3})^{1/2}$$$and its “bandpass” width, as a function of the control parameter $$$L_D=(TE \gamma^{2/3}G^{2/3}D_0^{1/3})^{1/2}$$$.
Results
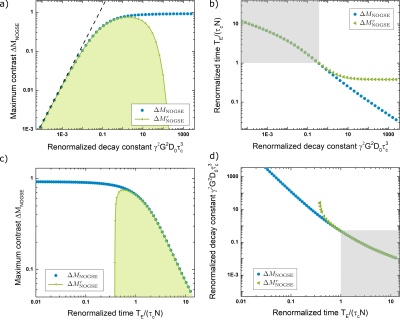
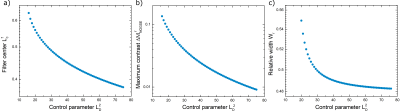
Figure 2.a-b shows the maximum $$$\Delta M_{NOGSE}$$$ and the renormalized time $$$\frac{TE}{\tau_cN}$$$ when it is achieved, as a function of $$$\gamma^2 G^2 D_0 \tau_c^3 $$$ for the full analytical expression $$$\Delta M_{NOGSE}$$$ and its first restricted approximation $$$\Delta M^r_{NOGSE}$$$ (reached by the restriction in Hahn) . This shows the parameter range where $$$\Delta M_{NOGSE}$$$ provides information on restriction sizes. The best tradeoff between the highest sensitivity towards restriction and signal contrast for a restriction length $$$l_c$$$ is achieved by setting $$$\gamma^2 G^2 D_0 \tau_c^3 \approx 1$$$ and $$$\frac{TE}{\tau_cN}$$$. Similarly, Fig.2.c-d shows the maximum $$$\Delta M_{NOGSE}$$$ and $$$\gamma^2 G^2 D_0 \tau_c^3$$$ where it is achieved as a function of $$$\frac{TE}{\tau_cN}$$$.Figure 3. a shows the $$$\Delta M^r_{NOGSE}$$$ filter center $$$L^f_c$$$ as a function of the control parameter $$$L_D$$$. To reduce the filtered size, $$$L_D$$$ must be increased. Increasing $$$L_D$$$ decreases the filtered signal amplitude (Fig.3.b). The minimum filtered size that can be attained is determined by the trade-off of reducing its size and holding a high enough SNR. The selectivity of the filter increases with $$$L_D$$$ (Fig.3.c). However, the selectivity does not change significantly for all the dynamic range of $$$L_D$$$, therefore SNR can be increased by reducing $$$L_D$$$ without losing selectivity.
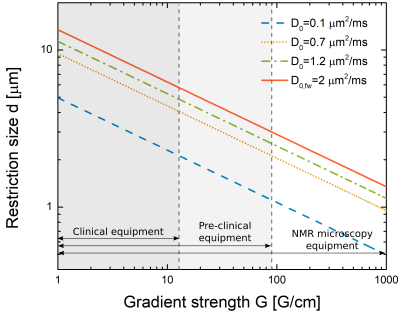
Assuming a typical cylindrical geometry (eg. axons), Figure 4 shows the minimum diameter that can be filtered for a range of control parameters found on clinical, pre-clinical, and research state-of-the-art scanners. The minimum size also depends on the diffusion coefficient. For typical white and gray matter diffusion coefficients, the filter would extract information on changes in the average sizes of the axons. This thus shows that current scanners can filter in diameters of a few microns for generating image contrast based on selective filtering microstructural sizes, and therefore probe microstructural features with just two measurements9.
Conclusions
By evaluating the NOGSE contrast $$$\Delta M_{NOGSE}$$$ as a function of control parameters, we found that restricted diffusion can be attained with maximum contrast by setting $$$\gamma^2 G^2 D_0 \tau_c^3 \approx 1$$$ and $$$\frac{TE}{\tau_cN}$$$. This defines the NOGSE limit for generating selective microstructure filters. We characterized NOGSE filter features of microstructural sizes, finding the minimum diameter that can be filtered depending on the sequence control parameters. This shows that microstructure sizes between 5-10[$$$\mu$$$m], e.g. axonal diameter, can be filtered using current scanner technologies.Acknowledgements
This work was supported by CNEA; CONICET; ANPCyT-FONCyT PICT-2017-3156, PICT-2017-3699, PICT-2018-4333; PIP-CONICET (11220170100486CO); UNCUYO SIIP Tipo I 2019-C028, 2022-C002, 2022-C030; Instituto Balseiro; A collaboration program from MINCyT (Argentina) and MAECI (Italy) and Erasmus+ Higher Education program from the European Commission between the CIMEC (University of Trento) and the Instituto Balseiro (Universidad Nacional de Cuyo). A.Z. and G.A.A. are members of the Research Career of CONICET. M.C. acknowledge support from the Instituto Balseiro fellowship.References
1. Padhani AR, et al.,Diffusion-weighted magnetic resonance imaging as a cancer biomarker: Consensus and recommendations, Neoplasia 11, 102 (2009).
2. Drago V., et al. Disease tracking markers for alzheimer’s Disease at the prodromal (MCI) stage. J. Alzheimers Dis. 26, 159 (2011).
3. Callaghan P. Translational Dynamics Magnetic Resonance, Principles of Pulsed Gradient Spin Echo NMR (Oxford University Press, Oxford, 2011).
4. White NS, et al. Probing tissue microstructure with restriction spectrum imaging: Histological and theoretical validation. Hum. Brain Mapp. 34, 327 (2013).
5. White NS, et al. Diffusion-weighted imaging in cancer: Physical foundations and applications of restriction spectrum imaging, Cancer Res. 74, 4638 (2014).
6. Alexander DC, Dyrby TB, Nilsson M, Zhang H. Imaging brain microstructure with diffusion mri: Practicality and applications. NMR Biomed. 32, e3841 (2019).
7. N. Shemesh, G. A. Álvarez and L. Frydman, Measuring small compartment dimensions by probing diffusion dynamics via Non-uniform Oscillating-Gradient Spin-Echo (NOGSE) NMR, J. Magn. Reson. 237, 49 (2013).
8. G. A. Álvarez, N. Shemesh and L. Frydman, Coherent Dynamical Recoupling of Diffusion-Driven Decoherence in Magnetic Resonance, Phys. Rev. Lett. 111, 080404 (2013).
9. M. Capiglioni, A. Zwick, P. Jiménez, and G. A. Álvarez, Noninvasive Quantitative Imaging of Selective Microstructure Sizes via Magnetic Resonance, Phys. Rev. App. 15(1), 014045 (2021).
10. A. Zwick, D. Suter, G. Kurizki and G. A. Álvarez. Precision Limits of Tissue Microstructure Characterization by Magnetic Resonance Imaging. Phys. Rev. Applied 14, 024088 (2020).
11. J. Stepisnik et al., Spectral characterization of diffusion in porous media by the modulated gradient spin echo with CPMG sequence, Journal of Magnetic Resonance 182, 195 (2006).
Figures