0761
Circular Echo-Planar Time-resolved Imaging (cEPTI) for Rapid Time-Resolved and Quantitative Imaging1Stanford University, Stanford, CA, United States, 2University of Southern California, Los Angeles, CA, United States
Synopsis
EPTI is a highly accelerated time-resolved imaging method for rapid quantitative imaging. To improve the spatiotemporal encoding efficiency of EPTI, we developed a “circular” EPTI sampling trajectory (cEPTI), designed to efficiently traverse a tight circular k-space coverage with full ramp-sampling. A systematic approach was also developed to characterize the noise and effective resolution of the EPTI acquisition and used to guide the optimization of cEPTI’s k-t sampling trajectory. The optimized cEPTI was demonstrated to be capable of producing a 50ms time-resolved series of distortion-free sharp brain images with varying T2* weightings at 1 mm in-plane resolution from a 195ms scan.
Introduction
Echo planar time-resolved imaging (EPTI) is a distortion-free multi-shot EPI technique for quantitative imaging1-2. It has been applied to the T1, T2, T2* mapping, diffusion, and myelin water imaging of brain with highly accelerated acquisition3-5. Recently, a circular EPI (cEPI) sequence has been developed, traversing a tight circular k-space coverage to reduced readout length6. Inspired by cEPI, in this work, we designed a novel circular EPTI (cEPTI) technique by incorporating the circular k-space coverage into the EPTI framework to reduce the readout echo spacing and thus reduce the number of EPTI-shots needed for k-t encoding to further accelerate the scan. The g-factor7-8 and PSF9 of subspace-based reconstruction were evaluated for existing EPTI and the proposed cEPTI techniques to characterize noise amplification and effective spatial resolution, respectively, providing guidance for cEPTI optimization. The optimized cEPTI achieved time-resolved distortion-free sharp imaging at 195ms for a single slice.Methods
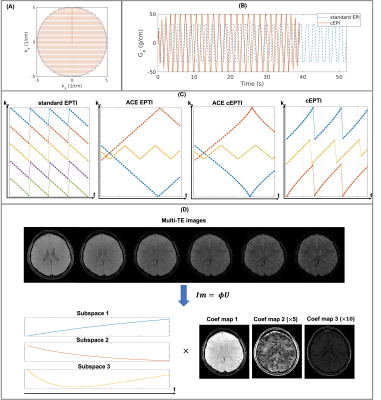
Sampling design for cEPTI: In cEPTI sequence, the maximum frequency encoding ($$$k_{x}^{max}$$$) is a function of phase encoding step with circular modulation, resulting in an increasing reduction of the echo spacing from center to outer k-space (Figure 1A). Full ramp-sampling was enabled to reduce the echo spacing by the maximum amount. As demonstrated in Figure 1B, the readout using cEPTI to traverse the entire k-space is only 75% of standard EPI, allowing cEPTI to achieve good k-space coverage with fewer shots. Figure 1C illustrates the ky-t sampling design for cEPTI. For comparison, the standard 5-shot EPTI and the 3-shot accelerated-echo-train shifted EPTI (ACE-EPTI) were also included. Two types of cEPTI schemes were designed: i) to match ACE-EPTI, referred to as ACE-cEPTI, and ii) to match standard EPTI, referred to as cEPTI. A 2D gradient echo sequence was used followed by different EPTI sampling patterns.Image reconstruction: The low-rank subspace reconstruction10 was used to exploit the high image correlation along the temporal dimension (Figure 1D). The subspace $$$\mathbf{\Phi}$$$ is pre-determined from a dictionary containing a range of physiological T2* values. The image is recovered using:
$$\widehat{\mathbf{U}}=\arg\min_{\mathbf{U}}\|\mathbf{MFSB}\mathbf{\Phi}\mathbf{U}-\mathbf{d}\|_{2}^{2}+LLR(\mathbf{U}), (1)$$
With acquired k-space data $$$\mathbf{d}$$$, undersampling mask $$$\mathbf{M}$$$, Fourier transform $$$\mathbf{F}$$$, coil sensitivity $$$\mathbf{S}$$$, B0 induced phase $$$\mathbf{B}$$$, coefficient maps $$$\mathbf{U}$$$, and locally low rank (LLR) regularizer.
The reconstruction quality is highly dependent on the pre-estimated B0 map. In this work, an initial B0 map is self-estimated from EPTI-data from an initial time-resolved sliding-window reconstruction across the EPTI-readout. Following that a B0-update scheme as described in a previous work2 was performed for a refined B0 map.
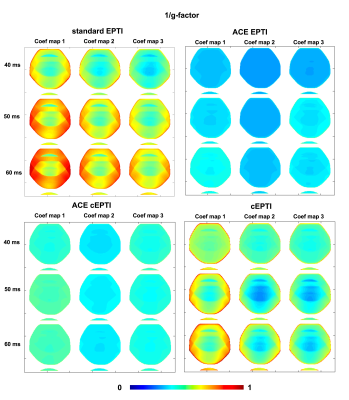
Theoretical characterization: g-factor7,8 and PSF9 are used to characterize the noise amplification and the effective resolution of the imaging system. In this work, the g-factor $$$g$$$ of the coefficient maps were evaluated for the four different sampling patterns at readout length of 40ms, 50ms, and to 60ms using the following equation:
$$g=\frac{1}{\sqrt{R}}\frac{\left(A_{a}^{H}\Psi^{-1}A_{a}\right)^{-1}}{\left(A_{f}^{H}\Psi^{-1}A_{f}\right)^{-1}}, (2)$$
with undersampling factor $$$R$$$, noise covariance $$$\Psi$$$, undersampling imaging model $$$A_{a}=\mathbf{MFSB}\mathbf{\Phi}$$$ and full-sampling imaging model $$$A_f=\mathbf{FSB}\mathbf{\Phi}$$$.
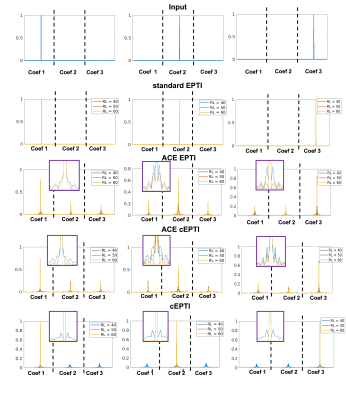
The PSF $$$x_{a}$$$ from a point source input $$$\delta$$$ is characterized as:
$$\widehat{x_{a}}=\underset{x_{a}}{\operatorname{argmin}}\left\|A_{a}\left(x_{a}\right)-A_{a}(\delta)\right\|^{2}, (3)$$
Simulations: A set of realistic proton density, T2*, sensitivity, and B0 map at 1-mm resolution were used to generate 2D time-resolved images with different readout length. After transforming to k-space, complex gaussian white noise were added to reach the SNR of 40 for the first echo time. Different undersampling patterns were performed and the images were reconstructed using Equation 1. The error between reconstructed images and ground truth were evaluated.
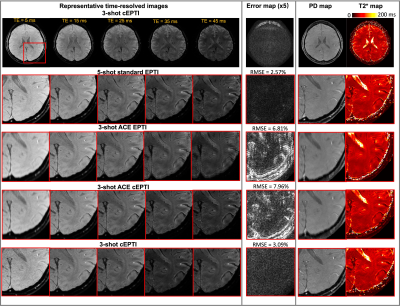
Imaging experiments: The in vivo studies performed on a 3T system (Premier, GE healthcare) with a 48-channel head coil. All the images were acquired in axial orientation using the following parameters: FOV=220x220mm2, spatial-resolution=1.0x1.0mm2, slice-thickness=5mm, number-of-slice=8, TE=5ms, TR=2000ms, readout=50ms, flip-angle=90°, with fat-saturation and RF-excitation time of 15ms. For standard and ACE-EPTI, the echo spacing is 1.1ms, resulting in 45 echoes per shot. For ACE-cEPTI and cEPTI, the longest echo spacing is 1.0ms and shortest echo spacing is 0.3ms.
Results
Figure 2 shows the 1/g-factor of the coefficient maps for different sampling patterns at 40ms, 50ms and 60ms. The g-factor of cEPTI at 50ms (scan time is 3*(50+15)=195ms) is approaching the g-factor from 5-shot EPTI at 40ms (scan time is 5* (40+15)=275ms, i.e. 41% longer), indicating that cEPTI is capable of achieving good conditioning at reduced encoding-shots and scan time.Figure 3 illustrates the PSF for different sampling schemes. When readout >= 50ms, cEPTI is able to recover an artifact/blur-free PSF, while ACE-EPTI and ACE-cEPTI showed blurry PSF with energy leakage into other coefficients.The simulation results are shown in Figure 4. The ACE-EPTI and ACE-cEPTI produced blurry images due to the PSF-blur and leakage, while cEPTI can recover sharp images with small noise-like error (RMSE=3.09%). Figure 5 is an initial in vivo demonstration of cEPTI. The images at different echo times, proton density and R2* map for each sampling scheme were evaluated. cEPTI produced dsharp image within 3 shots.Conclusion
A novel cEPTI technique was developed to further accelerate time-resolved brain imaging. The theoretical characterization, simulation, and in vivo results demonstrated that cEPTI is capable of retaining good SNR and image sharpness with a 3-shot acquisition of 195ms for a single high-resolution slice.Acknowledgements
References
1. Wang, Fuyixue, Zijing Dong, Timothy G. Reese, Berkin Bilgic, Mary Katherine Manhard, Jingyuan Chen, Jonathan R. Polimeni, Lawrence L. Wald, and Kawin Setsompop. "Echo planar time‐resolved imaging (EPTI)." Magnetic resonance in medicine 81, no. 6 (2019): 3599-3615.
2. Dong, Zijing, Fuyixue Wang, Timothy G. Reese, Berkin Bilgic, and Kawin Setsompop. "Echo planar time‐resolved imaging with subspace reconstruction and optimized spatiotemporal encoding." Magnetic resonance in medicine 84, no. 5 (2020): 2442-2455.
3. Wang, F., Z. Dong, T. G. Reese, L. L. Wald, and K. Setsompop. "3D‐EPTI for ultra‐fast multi‐contrast and quantitative imaging." Proc Intl Soc Mag Reson Med. Montreal(2019): 944.
4. Dong, Zijing, Fuyixue Wang, Lawrence Wald, and Kawin Setsompop. "SNR-efficient distortion-free diffusion relaxometry imaging using ACcelerated Echo-train shifted EPTI (ACE-EPTI)." bioRxiv (2021).
5. Dong, Zijing, Fuyixue Wang, Kwok-Shing Chan, Timothy G. Reese, Berkin Bilgic, José P. Marques, and Kawin Setsompop. "Variable flip angle echo planar time-resolved imaging (vFA-EPTI) for fast high-resolution gradient echo myelin water imaging." NeuroImage 232 (2021): 117897.
6. Rettenmeier, Christoph, Danilo Maziero, Yongxian Qian, and V. Andrew Stenger. "A circular echo planar sequence for fast volumetric fMRI." Magnetic resonance in medicine 81, no. 3 (2019): 1685-1698.
7. Pruessmann, Klaas P., Markus Weiger, Markus B. Scheidegger, and Peter Boesiger. "SENSE: sensitivity encoding for fast MRI." Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 42, no. 5 (1999): 952-962.
8. Wang, Jiayang, and Justin P. Haldar. "Transform-Domain g-Factor Maps." Proc. Intl. Soc. Mag. Reson. Med. 28 (2020)
9. Lustig, Michael, David Donoho, and John M. Pauly. "Sparse MRI: The application of compressed sensing for rapid MR imaging." Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 58, no. 6 (2007): 1182-1195.
10. Liang Z-P. Spatiotemporal imagingwith partially separable functions. IEEE; 2007:988-991.
Figures