3340
A universal framework to build digital reference objects for evaluation of quantitative MRI analysis with multiple measurements1Imaging Physics, MD Anderson Cancer Center, Houston, TX, United States
Synopsis
We propose a framework to easily design and generate digital reference object (DRO) s that conforms with DICOM standard for testing quantitative MRI analysis. The method focuses on using existing phantom scan as the basis and allows user to customize the phantom’s geometry and signal model. We generated a number of DRO for T2* and DSC quantitation and analyzed them with two different software as proof-of-concept.
Introduction
The accuracy of quantitative MRI methods depends on both the acquisitions and the post-processing analyses methods. An in silico digital reference object (DRO) can be used to validate and compare post-processing analyses methods without the difficulties and heterogeneities of in vivo or phantom acquisition1-3. Here, we propose a framework to easily design and generate DROs that conforms with DICOM standard and is vendor agnostic for testing quantitative MRI analysis.Methods
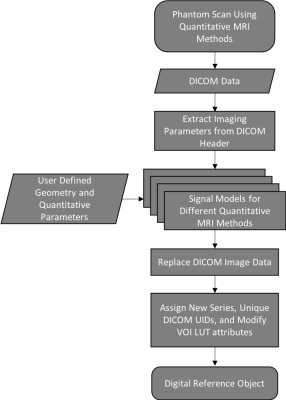
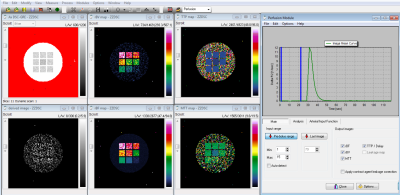
The study focuses on generating DROs from existing phantom scans with combinations of background object and test objects that can be selected from preset lists and customized. The general workflow for the framework in shown in Figure 1 and outlined in detail below: (1) If no prior phantom scans exist, phantom scans are acquired using quantitative MRI methods. (2) The user loads the DICOM data andspecifies (2.a) the background shape (ex. sphere, circle, Shepp-Logan) and basic geometry parameter (ex. radius), (2.b) the number and shape of test objects (ex. spheres, cylinders, cuboids) and basic geometry parameters (ex. radius, length, width, height), (2.c) the quantitative parameters for the signal model (ex. T2*, rCBF), and (2.d) the SNR. (3) The framework generates the DRO using both the user input and imaging parameters derived from the DICOM header. (4) The existing DICOM image data is replace with the DRO and the DICOM header is assigned a new series and unique DICOM UIDs, plus relevant modification such as the VOI LUT attributes (ex. window/level). The current prototype is implemented using Matlab 2018b (Natick, MA). To test the framework’s feasibility, several DROs were generated for T2* mapping and DSC MRI. They were loaded and analyzed with different FDA-cleared clinical software.Results
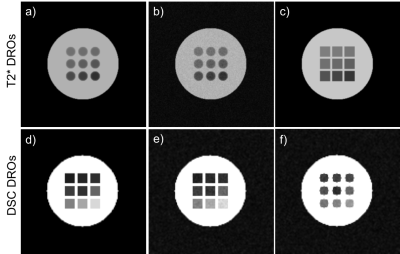
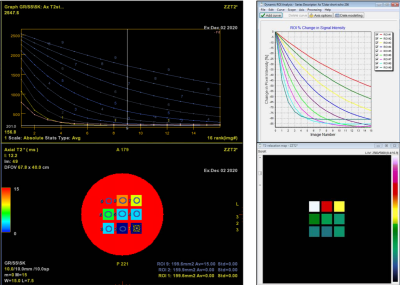
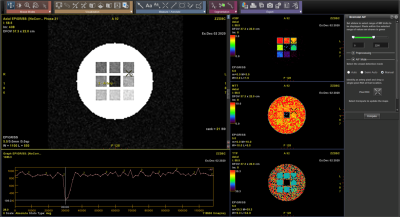
Examples of T2* and DSC DROs generated using the framework are shown in Figure 2 and were successfully analyzed by two software (Software 1 and Software 2). An example comparison of the performance of T2* quantitation between the two software using the T2* DRO from Figure 1c) is shown in Figure 3. Figure 4 and 5 shows the DSC DRO from Figure 1e). Both T2* mapping and DSC-MRI DROs held imaging parameters and other needed information in the DICOM metadata and were compatible with both software and processed without introducing error messages.Discussion
The framework presented in the abstract allows the generation of DROs based on existing phantom scans. This not only have the potential to allow the validation and comparison of pos-processing analyses methods, but to also generate DROs that accurately incorporate relevant imaging parameters of the quantitative MRI scans. The current software is command line only. We expect to build a graphical interface and provide more signal model modules to widen the frameworks applicability.Conclusion
Our framework for generating DRO that utilizes existing phantom scans provides an easy way to design and generate DICOM conformant DROs that is vendor agnostic for testing quantitative MRI analyses.Acknowledgements
No acknowledgement found.References
1. Bosca RJ, Jackson EF. Creating an anthropomorphic digital MR phantom—an extensible tool for comparing and evaluating quantitative imaging algorithms. Physics in Medicine & Biology. 2016 Jan 7;61(2):974.2.
2. Semmineh NB, Stokes AM, Bell LC, Boxerman JL, Quarles CC. A population-based digital reference object (DRO) for optimizing dynamic susceptibility contrast (DSC)-MRI methods for clinical trials. Tomography. 2017 Mar;3(1):41.3.
3. Bell LC, Semmineh N, An H, Eldeniz C, Wahl R, Schmainda KM, Prah MA, Erickson BJ, Korfiatis P, Wu C, Sorace AG. Evaluating multisite rCBV consistency from DSC-MRI imaging protocols and postprocessing software across the NCI quantitative imaging network sites using a digital reference object (DRO). Tomography. 2019 Mar;5(1):110.
Figures