3072
Isotropic T1-mapping of the whole brain by MP-RAGE at different inversion times1Medical Radiation Physics, Clinical Sciences Lund, Lund University, Lund, Sweden
Synopsis
Fitting the inversion recovery (IR) at multiple TI provides T1 estimates of high accuracy if performed in a single slice with full relaxation. We present an isotropic 3D variant based on MP-RAGE, where a T1-weighted driven equilibrium is prepared prior to inversion by a second RAGE readout. This abolishes the need for long recovery times and provides volumes of similar contrast for co-registration. The method was tested at 3T on a small cohort and compared to 3D IR-TSE and 3D variable flip angle mapping.
Introduction
Fitting the exponential inversion recovery (IR) at multiple TI at full relaxation provides T1 estimates of high accuracy since this excludes bias by inhomogeneous B1+1. Since this is typically performed in a single slice and due to time requirements, this is mainly used to validate other T1 mapping methods. A driven equilibrium (DE) is reached much faster than M0 by relaxation, thus accelerating IR measurements when combined with non-equilibrium rapid acquisition of gradient echoes (RAGE). By using the second volume of MP2RAGE2 to establish a DE, isotropic three-dimensional (3D) IR fitting of T1 becomes possible.Theory
At the n+1-th readout by flip angle α during RAGE at TR, Mz has converged on the DE at M0(1-exp(R1TR))/(1-exp(R1*TR)) by exp(-R1*nTR) = exp(-R1nTR)*cosnα, featuring a relaxation and a driving term3. While small α and small n partially maintain the dynamic range of the IR-prepared Mz, a large enough α are required to reach the DE for a given turbo-factor. These conflicting demands can be met by exploiting the two read-out trains of MP2RAGE.Methods
Measurements were performed on a 3T MAGNETOM Prisma using a 20 channel head-neck coil (Siemens Healthcare, Erlangen, Germany) on informed consenting healthy adults (3m/2f, 26-53years). Sagittal non-selective volumes of 1.25 mm3 isotropic resolution (FoV 240x232x180 mm3) were acquired using an MP2RAGE at TE/TR/α1/α2 =2.6/5.6ms/5°/20° while varying TI through 300ms, 600ms, 1000ms, 1600ms, 2500ms and 4000ms. The turbo-factor was 144. At 20°, Mz converges by more than 1% (for a typical lower B1+=75%). With 6/8 partial Fourier acquisition (n=48) and α1=5° more than 75% of the dynamic range from IR-preparation are preserved (driving term only, at upper B1+=125%). The additional time to establish DE was 850ms. Without delay between the second RAGE and the inversion, the measurement time per volume was between 2:22 and 7:32 minutes. Volumes were co-registered using the T1-w DE volumes. T1 was obtained by fitting an exponential transition to the first MP-RAGE volumes. For comparison, T1 was also mapped at five variable flip angles (4°, 6°, 8°, 12° and 16° at constant B1= 8.16uT, TR = 10ms)4, corrected by the scanner's default saturation recovery B1+ maps5 in one subject and by fully relaxed 2D IR-TSE in another.Results
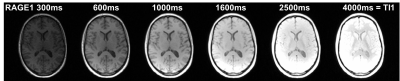
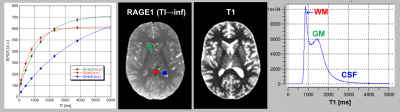
For different TI, the first MP-RAGE volumes showed a strong dependence on TI (Figure 1). The similar contrast of the other volume (not shown) indicated convergence onto the T1-weighted DE. Thus, Mz was positive even at the shortest TI. Fit to ROI signals (Fig. 2) resulted in small residues with R close to 1 as typical for IR. At TI=4000ms, the endpoint was attained only in white matter.For the standard locations in splenium, caudate and lateral ventricle, values obtained from 3D MP-RAGE and 2D TSE agreed well: 866±44ms/870±51ms, 1337±52ms/1417±16ms and 4179±1265ms/3858±317ms. Corresponding cohort means±SD from 3D MP-RAGE were 871±43ms, 1432±80ms, and 4294±171ms, respectively.
The poorer definition of the endpoint with increasing T1 explains the loss of precision as seen in the scatterplot (Fig. 3). This could be ameliorated by increase the longest TI value (and omitting some shorter TI). Here, VFA and DE-MP-RAGE yielded T1 of similar precision. Linear regression revealed a slight underestimation of T1 by VFA (as explained by inverse magnetization transfer)6.
Discussion
By turning MP2RAGE into a DE-IR-prepared measurement, we obtained 3D T1 maps in only slightly more time than by the (gold-)standard 2D IR-prepared TSE. This is possible due to the RAGE readout and the preparation of DE taking less time than restoring M0 by relaxation. This comes at a cost of reducing the dynamic range of the IR-prepared state.Details of our implementation depended on the MR system’s MP2RAGE sequence, 20° being the highest possible α2. Complete saturation of Mz can be efficiently achieved by replacing RAGE by additional 90° pulses. Although α2 = 5° was the Ernst angle in GM, higher SNR may be achieved by increasing α1, but at a cost of dynamic range. The method is applicable at 7T (data not shown). The DE-IR-prepared T1 maps are intended to serve as reference for 3D T1 mapping methods that may be subject to spatial bias, e.g. to validate the reduced B1+ sensitivity of T1 mapping by MP2RAGE.2
Conclusion
Preparing a DE prior to inversion with an MP2RAGE sequence allows for isotropic T1 mapping of the whole brain, thus providing a B1+-independent, IR-based T1 reference to validate T1 quantification methods.Acknowledgements
Funding by the Swedish Research Council (NT-2014-6193) and support by Dr. F. Testud, Siemens Healthcare Sweden, is gratefully acknowledged.References
1) Kingsley PB, Monahan WG. Effect of increased repetition time TR on precision of inversion-recovery T1 measurements. Magn Reson Imaging 2001;19:279–282.
2) Marques JP, Kober T, Krueger G, van der Zwaag W, Van de Moortele PF, Gruetter R. MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. Neuroimage 2010;49:1271-1281.
3) Deichmann R, Good CD, Josephs O, Ashburner J, Turner R. Optimization of 3-D MP-RAGE sequences for structural brain imaging. NeuroImage 2000;12:112-127.
4) Helms G, Dathe H, Weiskopf N, Dechent P. Identification of signal bias in the variable flip angle (VFA) method by linear display of the algebraic Ernst equation. Magn Reson Med 2011;66:669-77.
5) Chung S, Kim D, Breton E, Axel L. Rapid B1+ mapping using a preconditioning RF pulse with TurboFLASH readout. Magn Reson Med 2010;64:439-46.
6) Al-Abasse Y, Helms G. Influence of pulse length and shape on variable flip angle T1 mapping of the human brain. Proc ISMRM 2016 24:696.
Figures