2516
Imaging performance of a multi-channel non-human primate coil1Wellcome Centre for Integrative Neuroimaging, FMRIB, Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford, United Kingdom, 2Wellcome Centre for Integrative Neuroimaging, Department of Experimental Psychology, University of Oxford, Oxford, United Kingdom, 3Wellcome Centre for Integrative Neuroimaging, OHBA, Department of Psychiatry, University of Oxford, Oxford, United Kingdom, 4British Heart Foundation Centre of Research Excellence, Department of Cardiovascular Medicine, University of Oxford, Oxford, United Kingdom
Synopsis
A 15-channel array coil has been developed for our centre by a coil manufacturer for the purposes of awake behaving macaque fMRI. Here, we evaluated the imaging performance in vivo and on phantoms. Compared to a 4 channel flexible coil, we found significant improvements in SNR and tSNR both in vivo and in phantom studies. Furthermore, we demonstrate the feasibility of multiband and in-plane accelerated acquisitions, up to a multiband factor of 6 with in-plane acceleration of 2.
Introduction
The functional and structural imaging of non-human primates offers valuable insights into the neuroanatomy and brain development of humankind's closest relatives1. However, such imaging requires specialised coils2-4, particularly for awake behaving fMRI, and bespoke hardware for animal handling. Here, we characterise the imaging performance of a bespoke, 15-channel coil (Rapid Biomedical, Rimpar, Germany) developed for our centre to be suitable for awake behaving fMRI of rhesus macaques in the sphinx position, and compare it to a 4-channel flexible coil5 currently in use. Crucially, the 15-channel coil is designed for highly temporal resolution functional imaging via multiband acceleration.Design Considerations
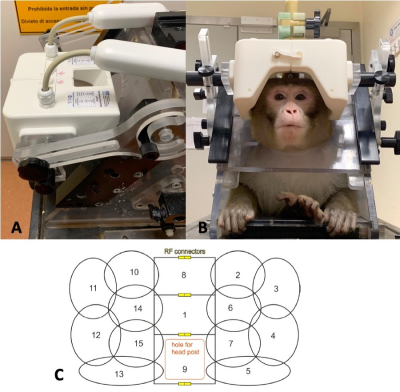
Head size, method of head restraint, animal handling and desired imaging performance placed constraints on the geometry on the novel coil. Within our centre, 3T functional MRI is carried out on awake, behaving rhesus macaques positioned in a special chair (Rogue Research, Montreal, CA), sitting in the sphinx position. The 15-channel coil had to fit with this setup. Furthermore, head size varies greatly with age and sex, and the largest head was chosen as a constraint.Animal heads are fixed in position using a headpost implanted onto the parietal bone, and stimulus is presented at the end of the bore. The coil had to accommodate a head post hole while covering the back of the head, not obscure vision, and allow for animal handling during preparation.
We aimed to achieve imaging benchmarks of an in-plane parallel acceleration factor of at least 2, and the capability to simultaneously enable multiband imaging in the head-foot direction.
Consequently, the coil consists of fifteen partially overlapping receive-only loops tuned to 123.2 Mhz, arranged in two halves split along the AP axis, with three loops split. A hole in the coil housing allows for head fixation. The interior surface of the coil allows for large head sizes of 140mm in the right-left and 130 mm in the anterior-posterior direction.
Methods
Acquisitions: All scans were acquired on a 3T scanner, using the 15ch coil (Rapid Biomedical, Rimpar, Germany) and a 4ch flexible phased-array coil with radial local transmit (Windmiller KolsterKostler Scientific, Fresno, CA, USA). All images were reconstructed using stock, vendor-provided reconstruction. Phantom study: A cylindrical phantom (11cm diameter, 22cm height, 3.75g NiSO4+5gNaCL/1000g) was scanned using both coils. An MPRAGE sequence (TR/TE=1580/4, FA=8, resolution=0.5mm isotropic, FOV=176x176x128mm, no acceleration), and a GRE-EPI sequence (TR/TE=2410/30ms, FA=90deg, resolution=1.5mm isotropic, FOV=130x130mm in-plane, 36 slices, GRAPPA acceleration 2) were acquired. In-vivo study: A GRE-EPI sequence with matching parameters to the phantom study was acquired on a male rhesus macaque (Maq1, 6 years, 11.4 kg) using the 15ch coil, and a male rhesus macaque (Maq2, 8 years, 14.8kg) using the 4ch flexible coil. On Maq1, a series of multiband sequences (50 volumes) with resolution and coverage matching that of the GRE-EPI sequence, using the shortest possible TR, were acquired as a proof of feasibility. All multiband sequences were acquired using in-plane acceleration factor of 2, and TR/TE were 2280/30ms for multiband factor 1 (no acceleration); 1120/30ms for multiband factor 2; 633/30ms for multiband factor 4; and 500/30ms for multiband factor 6, respectively. All procedures were conducted under licences from the United Kingdom Home Office in accordance with the UK Animals Act 1986 and by the University of Oxford Animal Care and Ethical Review Committee. Analysis: SNR and tSNR were derived from reconstructed images, noise correlation was estimated from thermal noise data.Results
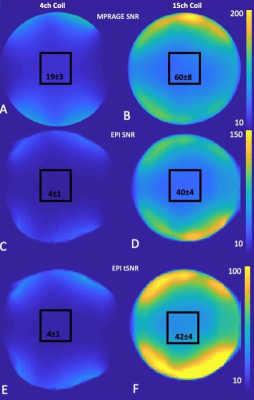
Figure 1 shows the design of the coil. A range of head sizes is accommodated, vision is not impaired.Figure 2 shows the SNR and tSNR maps between the 15-channel coil and the 4-channel flexible coil. Within the central volume (black square), SNR gain was approximately threefold for MPRAGE and approximately ninefold for EPI scans. tSNR gain was ninefold.
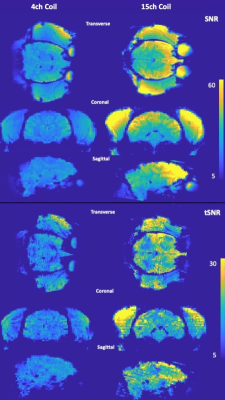
Figure 3 shows the SNR and tSNR maps between the 15-channel coil and the 4-channel flexible coil in vivo. While a like-for-like comparison was not possible, the SNR and tSNR grain (approximately 150% and 130% in the centre of the brain, respectively) is notable.
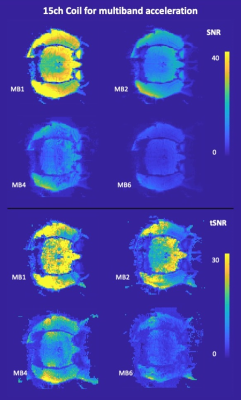
Figure 4 shows SNR and tSNR maps for the multiband scans using the 15-channel coil. Within the brain, SNR was 23∓3 for MB1, 10∓1 for MB2, 8∓2 for MB4 and 4∓1 for MB6, while tSNR was 20∓6 for MB1, 15∓3 for MB2, 9∓2 for MB4 and 4∓1 for MB6.
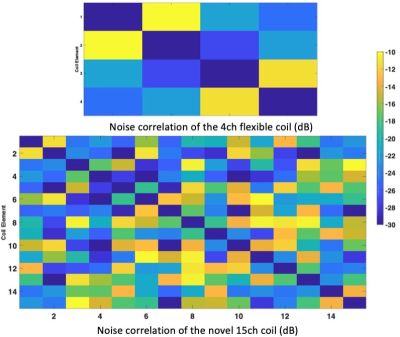
Figure 5 shows the noise correlation matrices for and phantom scans. All coil elements are decoupled with at least -10 dB.
Conclusion
A bespoke 15-channel headcoil for awake behaving macaque fMRI was designed and constructed based on our criteria, and evaluated for imaging performance both in vivo and phantom imaging. Compared to current, 4-channel flexible coils, the bespoke coil shows an increase in SNR and tSNR, and will enable our centre to carry out multiband accelerated fMRI scanning, generating functional data at much higher temporal resolution than previously possible. Multiband imaging is feasible up to a combined acceleration factor of 12 (R=2, MB=6), however stock reconstruction results are affected by physiological noise. Further work will focus on protocol development and bespoke reconstruction algorithms, to fully exploit the capabilities of the coil for neuroscience applications of accelerated awake behaving fMRI.Acknowledgements
We thank Rapid Biomedical for the construction and delivery of the novel coil.
The Wellcome Centre for Integrative Neuroimaging is supported by core funding from the Wellcome Trust (203139/Z/16/Z).
References
1: Grohn et al. (2020)PLoS Biol 18(10)
2: Mareyam et al. (2018) Proc. Intl. Soc. Mag. Reson. Med. 26:4280
3: Papoti et al. (2017) Magn Reson Med 78(1):387-398
4: Wei et al (2019) Proc. Intl. Soc. Mag. Reson. Med. 27:1608
5: https://www.wkscientific.com/#mri-coils
Figures