1847
Velocity-Selective Inversion prepared Arterial Spin Labeling: Examination in a Commercial Perfusion Phantom11The Russell H. Morgan Department of Radiology and Radiological Science, Johns Hopkins University, Baltimore, MD, United States, 2F.M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States, 3Biomedical Engineering, Johns Hopkins University, Baltimore, MD, United States
Synopsis
Standardized perfusion phantom allows for controlling reproducibility of arterial spin labeling (ASL) techniques across multiple sites, field strength, and vendors. Here using a commercial perfusion phantom, velocity-selective inversion (VSI) prepared ASL was compared with PCASL with an emphasis on velocity-encoding directions. While this effect is amplified on this phantom with feeding channels having only vertical and transverse flow directions, VSI-ASL with a tilted encoding direction achieved higher labeling efficiency through more uniform labeling of the entire feeding tubes. Careful selection of velocity-encoding directions along the major feeding arteries is recommended for VSASL applications to attain optimal labeling efficiency.
Introduction
Velocity-selective inversion (VSI) prepared VSASL, first demonstrated for measuring cerebral blood flow (CBF)1, have been applied successfully for perfusion mapping of different organs2-6. The labeling utility of the Fourier-Transform (FT) based VS pulse train was directly visualized in cerebral VSMRA7. It is well recognized that standardized perfusion phantoms, that provide predictable, reproducible, and quantifiable flow dynamics, would facilitate the utilization of advanced ASL techniques across multiple sites, field strength, and vendors8,9. A commercial perfusion phantom QASPER was recently introduced10. It has been quantitatively characterized by two common ASL methods, flow-sensitive alternating inversion recovery (FAIR)10 and pseudo-continuous ASL (PCASL)11-14. In this work, VSI prepared VSASL was compared with PCASL using QASPER at a 3T scanner.Methods
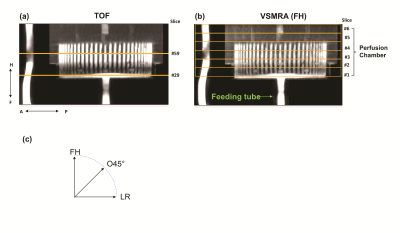
Experiments were conducted on a 3T Philips Ingenia scanner using a 32-channel head-only coil for signal reception. The specifics of the QASPER phantom are described online (goldstandardphantoms.com/qasper). The MRA results (detailed below) delineates the structure of the phantom (Figure 1a, b), which contains the main feeding tube within the label chamber (for PCASL) and the diverted 60 channels feeding the perfusion chamber with a stack of perfusion material consisting of six porous layers.Two controlled flow rates, first 350mL/min and then 175mL/min, were used in this experiment. 2D phase-contrast (PC) MRIs were acquired before and after the MRA and ASL scans to verify the delivered flow. At each flow rate, in addition to TOF and PCASL, FT-VSS based MRA and FT-VSI based VSASL were each performed with three velocity-encoding directions, foot-head (FH), left-right (LR), and oblique 45° on the coronal plane (O45°) (Figure 1c). The FT-VS pulse trains used in the current work was described previously (6).
The acquisition parameters of TOF and VSMRA were: FOV=180x180x40 mm3, acquired/ reconstructed resolution=0.7x0.7x1.0/0.35x0.35x0.5mm3, duration=3min.
Post labeling delay (PLD) of VSI-ASL was 1500ms (1,6). PCASL used labeling duration=1800ms and PLD=1800ms. The labeling plane was 60mm below the center of imaging volume. The acquisition parameters were 3D GRASE, FOV=180x180x30mm3, acquired/reconstructed resolution=1.8x1.9x5.0/1.4x1.4x5.0mm3, 6 slices, EPI factor=15, SENSE=2, TSE factor=6, shot duration=109 ms, TE=18ms, and TR=4.8s/5.0s for VSASL/PCASL. Three pairs of label and control scans were of 3.5 min. An M0 was acquired with the same parameters but TR of 10s.
Ring ROIs (Figure 4) were generated from ASL difference images to investigate the effect of angular directions. Two sectors of 45° on the left side and top were selected on slice#5 (Figure 4 marked by green and yellow lines) for comparing perfusion-weighted signal between flow rates and across methods. Slice #5 was above the 60 vertical arteriole channels at slice #4. CBF derived from PCASL at 350 (PCASL-350) mL/min used labeling efficiency of 0.85. As flow rate was known, labeling efficiency of all VSASL and PCASL-175 mL/min was determined by scaling their derived CBF to be equal to PCASL-350mL/min CBF.
Results and Discussion
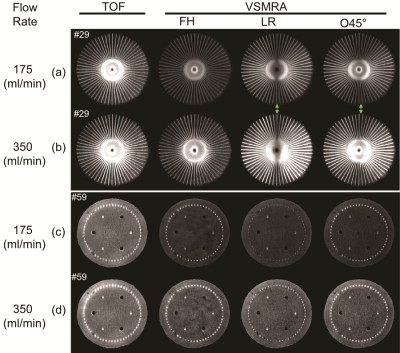
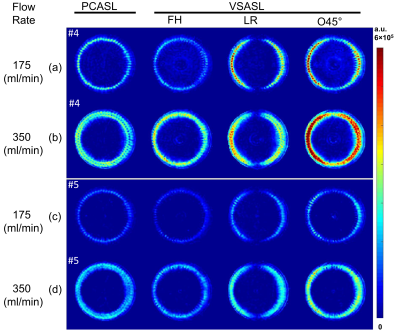
Figure 2 shows the comparison among TOF, VSMRA with FH, LR and O45° velocity-encoding directions at slices #29 and #59 for both 175 and 350 mL/min flow rates. VSMRA reveals the dependence on the velocity-encoding directions, such that signal is minimum when flow direction is orthogonal to the encoding direction. With O45°, the loss of signal along the LR direction is mitigated. There is a central dark band along AP direction due to its perpendicular direction to O45° (#29).Figure 3 shows the raw difference images among PCASL, VSASL with encoding directions of FH, LR and O45° at two flow rates. VSASL (FH) showed narrower perfusion ring than PCASL, as it missed about 500/1000ms bolus (for 350/175 mL/min) at the 60 transversal channels due to its poor labeling efficiency shown in Figure 2(a, b). Conversely, VSASL (LR) demonstrated higher perfusion signal at the left and right sides, as a result of the effective labeling bolus in the transversal channels along LR directions. VSASL (O45°) combines labeling bolus obtained by FH and LR, thus yielding strongest perfusion signal at the left and right sides.
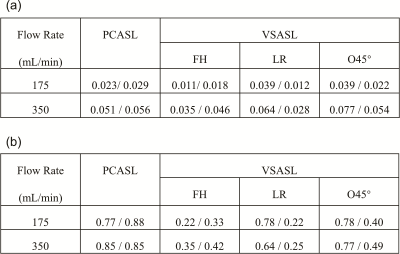
Perfusion signal as a function of angular direction was illustrated in Figure 4. Signal dips of LR and O45° at 90° reflects their direction dependence at the transversal plane. Averaged normalized signal (diff/M0) from the left and top sectors were listed in Table 1a. Perfusion signals of VSASL (O45°) at the left-side ROI at the 350mL/min flow rate are almost two times of the ones at the 175mL/min, like the PCASL results. By setting PCASL-350 mL/min as a reference, the labeling efficiency was calculated and listed in Table 1b. As expected, O45° left side had the highest labeling efficiency (~0.78) across flow rates and ROIs among VSASL methods.
Conclusion
Direction dependence of VSI prepared ASL is amplified on the QASPER phantom as the feeding channels have only vertical and transverse flow directions. When using a tilted encoding direction with more uniform labeling through the feeding tubes, VSI-ASL achieved higher labeling efficiency than using suboptimal encoding directions and yielded perfusion signals proportional to the controlled flow rate. Careful selection of velocity-encoding directions along the major feeding arteries is recommended for VSASL applications in various organs to attain optimal labeling efficiency.Acknowledgements
We want to thank Aaron Oliver-Taylor and Xavier Golay for providing guidance of using the QASPER phantom.References
1. Qin Q, van Zijl PC. Velocity-selective-inversion prepared arterial spin labeling. Magn Reson Med 2016;76(4):1136-1148.
2. Hernandez-Garcia L, Nielsen JF, Noll DC. Improved sensitivity and temporal resolution in perfusion FMRI using velocity selective inversion ASL. Magn Reson Med 2019;81(2):1004-1015.
3. Franklin SL, Bones IK, Harteveld AA, Hirschler L, van Stralen M, Qin Q, de Boer A, Hoogduin JM, Bos C, van Osch MJP, Schmid S. Multi-organ comparison of flow-based arterial spin labeling techniques: Spatially non-selective labeling for cerebral and renal perfusion imaging. Magn Reson Med 2020.
4. Guo J, Das S, Hernandez-Garcia L. Comparison of velocity-selective arterial spin labeling schemes. Magn Reson Med 2020.
5. Landes V, Javed A, Jao T, Qin Q, Nayak K. Improved velocity-selective labeling pulses for myocardial ASL. Magnetic Resonance in Medicine 2020;84(4):1909-1918.
6. Liu DP, Xu F, Li WB, van Zijl PC, Lin DRD, Qin Q. Improved velocity-selective-inversion arterial spin labeling for cerebral blood flow mapping with 3D acquisition. Magn Reson Med 2020;84:2512-2522. 7. Qin Q, Shin T, Schär M, Guo H, Chen H, Qiao Y. Velocity-selective magnetization-prepared non-contrast-enhanced cerebral MR angiography at 3 Tesla: Improved immunity to B0/B1 inhomogeneity. Magn Reson Med 2016;75(3):1232-1241.
8. Keenan KE, Ainslie M, Barker AJ, Boss MA, Cecil KM, Charles C, Chenevert TL, Clarke L, Evelhoch JL, Finn P, Gembris D, Gunter JL, Hill DLG, Jack CR, Jr., Jackson EF, Liu G, Russek SE, Sharma SD, Steckner M, Stupic KF, Trzasko JD, Yuan C, Zheng J. Quantitative magnetic resonance imaging phantoms: A review and the need for a system phantom. Magn Reson Med 2018;79(1):48-61. 9. Kamphuis ME, Greuter MJW, Slart R, Slump CH. Quantitative imaging: systematic review of perfusion/flow phantoms. Eur Radiol Exp 2020;4(1):15.
10. Oliver-Taylor A, Gonçalves M, Hampshire T, Davis B, Daga P, Evans L, Bainbridge A, Wheeler-Kingshott C, Sokolska M, Thornton J, De Vita E, Golay X. A Calibrated Perfusion Phantom for Quality Assurance of Quantitative Arterial Spin Labelling. ISMRM. Honolulu, Hawaii, USA. 2017. p 0681.
11. Golay X, Oliver-Taylor A, Susuzi Y, Chappell M. How to quantity ASL values in a perfusion phantom. ESMRMB. Rotterdam, Netherlands. 2019. p 1672.
12. Oliver-Taylor A, Hampshire T, Mutsaerts HJ, Clement P, Warnert E, Kuijer JPA, Baas K, Petr J, Siero JCW, Marques JP, Sunaert S, Borra RJH, van Osch MJP, Golay X, Achten E. A multi-site round robin assessment of ASL using a perfusion phantom. ISMRM. Montréal, QC, Canada. 2019. p 2653.
13. Petitclerc L, Teeuwisse WM, van Osch MJP. Characterization and Validation Protocol for a Perfusion Phantom used in Arterial Spin Labeling Experiments. ESMRMB. Rotterdam, Netherlands. 2019.
14. Warnert EAH, Steketee RME, Vernooij MW, Ikram MA, Vogel M, Tamames JAH, Kotek G. Implementation and validation of ASL perfusion measurements for population imaging. Magn Reson Med 2020;84(4):2048-2054.
Figures