1712
High-Resolution Post-Mortem Diffusion MRI Acquisitions for Connectivity Analyses in Chimpanzees1Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany, 2Alfred Wegener Institute Helmholtz Centre for Polar and Marine Research, Bremerhaven, Germany, 3Project Group Epidemiology of Highly Pathogenic Microorganisms, Robert Koch Institute, Berlin, Germany, 4Tai Chimpanzee Project, Centre Suisse de Recherches Scientifiques en Cote d'IVoire, Abidjan, Cote D'ivoire, 5Center for Cognitive Neuroscience Berlin, Freie Universität Berlin, Berlin, Germany, 6Max Planck Institute for Evolutionary Anthropology, Leipzig, Germany
Synopsis
Detailed neuroanatomical comparisons between humans and chimpanzees could greatly benefit evolutionary neuroscience. However, ethical considerations regarding primate research disallow acquisitions of chimpanzee MRI data in vivo for multiple years. Hence, the availability of diffusion MRI (dMRI) and tractography data from chimpanzees is limited to a few previously acquired datasets. Here we optimize diffusion acquisitions for an interdisciplinary approach to great ape neuroimaging, using post-mortem dMRI data from naturally deceased wild and captive animals. The optimization of data quality from two acquisition strategies allowed to us acquire chimpanzee diffusion MRI data of unpreceded quality and reopen a gateway for evolutionary neuroscience.
Introduction
The evolutionary origin of human brain function and its connectivity is not yet well understood. This knowledge gap may be closed by comparing human brain connectivity with that of great apes, (e.g., Chimpanzees) (1,2). However, ethical concerns about primate research prohibit neuroimaging research on great apes (3). Therefore, evolutionary neuroscience relies on a small number of previously acquired diffusion MRI (dMRI) data. We here present a novel approach for dMRI data acquisition in great apes, utilizing post-mortem chimpanzee brains from naturally deceased animals, which originate from African wildlife field-sites, sanctuaries, and European zoos. We optimize and compare the achievable dMRI quality for post-mortem dMRI acquisitions on a human and preclinical MRI system using two different sequences. The resulting data quality allowed an isotropic resolution of 500μm, to our knowledge the highest dMRI resolution yet achieved in chimpanzees.Methods
Tissue SamplesThe wild animal brains used for this research originated from African wildlife field-sites, where the individual behavior of the chimpanzees have been monitored without human interaction. Captive animal brains originated from African animal sanctuaries and European zoos. The brains were extracted after natural death within a post-mortem-interval of only 2-24h. The brains were immersion-fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS), shipped to Germany, washed in PBS and immersed in perfluoropolyether for scanning.
Pre-scan for Optimal Diffusion-Weighting
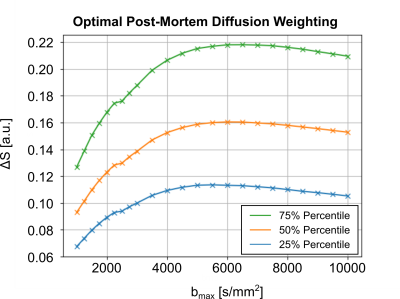
The required fixation of post-mortem tissue alters its tissue properties. Hence, the diffusion coefficients of fixed tissue are reduced by varying factors (4). The optimal diffusion-weighting of the tissue was determined using pre-scans, acquired using a 3T Connectom MRI System, equipped with a gradient strength of Gmax=300mT/m and a 32Ch head coil (Siemens Healthineers, Germany). Diffusion-weighted pre-scan acquisitions were acquired with a low isotropic resolution of 2mm in 22 diffusion shells (30 directions), ranging from b=1000s/mm2 to b=10000s/mm2. Diffusion-contrast was assessed per shell, as the diffusion signal difference between the main and perpendicular DTI orientation per voxel, ΔS. The diffusion-weighting with the highest median ΔS across the entire brain volume was chosen as optimal diffusion-weighting (Figure 1).
MRI Acquisitions and System Comparison
High-resolution dMRI sequences with optimal diffusion parameters and an isotropic resolution of 500μm were set upon two different MRI systems – a 3T Connectom and a 9.4T Bruker Biospec 94/30. Both systems feature a minimum 30 cm wide magnet-bore accommodating entire chimpanzee brains (5). Diffusion MRI sequences were optimized for high-resolution image quality under consideration of system-specific hardware constraints (6–9) (Figure 2). Reversed phase-encoding acquisitions were acquired for distortion correction (10). A noise map was acquired on both systems with matching acquisition parameters by turning off the RF excitation.
Data Processing
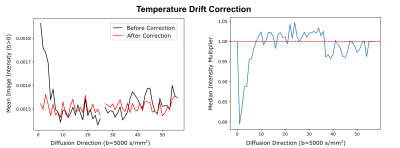
The SNR of the non-diffusion-weighted data was calculated for both systems in one brain in a voxel-wise manner, using the dMRI data and the noise map. The resulting SNR distribution was compared between both systems. The processing of the dMRI data included debiasing (11), denoising (12), temperature drift correction, and correction of sample movement and distortion (13). The temperature drift correction compensated for tissue diffusivity that increases with temperature over time, due to heating (Figure 3). For this purpose, a time-specific factor was calculated, which scales signal intensities to match steady-state temperature conditions. Initial tractography and visualization of the processed dataset were performed using brainGL.
Results
Optimal Diffusion-WeightingFigure 1 displays the post-mortem diffusion-contrast as a function of diffusion-weighting on a representative sample. Most samples indicated a consistent maximum contrast, ΔS, at b=5000s/mm2. Consequently, this diffusion-weighting was chosen to be used as the standard for dMRI acquisitions.
MRI System Comparison
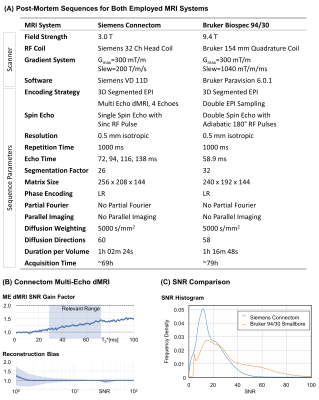
Figure 2A summarizes the sequences, employed for optimal data quality with 0.5mm isotropic resolution on both utilized MRI systems. The 3T Connectom post-mortem acquisition was optimized using a 3D version of the previously proposed Multi-Echo dMRI sequence (7) with four echoes. The noise-informed Multi-Echo reconstruction achieved an overall SNR gain of 25% with negligible Rician reconstruction bias (Figure 2B). The 9.4T preclinical acquisition utilized adiabatic refocusing to homogenize image intensity (7). Double EPI sampling was chosen to minimize ghosting (9). The SNR comparison indicated a higher SNR for the preclinical sequence (Figure 2C). Hence, the remaining dMRI were acquired with the preclinical MRI system.
Data Processing
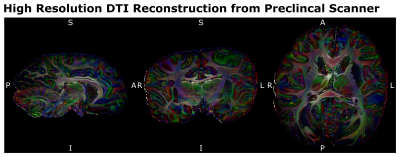
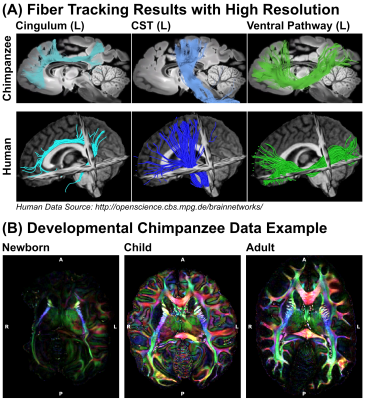
The processed dMRI data acquired on the 9.4T preclinical MRI system were summarized in Figures 4 and 5. The dMRI data were of unprecedented image resolution for whole chimpanzee brains and allow tractography reconstructions of chimpanzees from various ages.
Discussion
In this work, we describe the comparison of different MRI hardware and sequences to achieve high quality dMRI data of post-mortem tissue, which was noninvasively provided by a network of African and European collaboration partners. We describe a systematic approach to optimize diffusion-weighting for post-mortem data as well as a correction scheme for temperature effects in time-consuming post-mortem dMRI acquisitions. As a result, we obtained the highest resolution dMRI data ever collected on chimpanzees. The dMRI data allow tractography reconstructions to compare structural connectivity between chimpanzees and humans. The non-invasive selection of naturally deceased animals from the wild provides access to brains from all ages, thereby enabling novel access to the development of ape brain connectivity and a potential individual link between brain structure and behavior.Acknowledgements
This work was funded by the presidential funds of the Max Planck Society to the Inter-Institutional Research Initiative ‘Evolution of Brain Connectivity’.
The Max Planck Society also provides core funding for the Taï Chimpanzee Project since 1997.
We thank the Ministère de l’Enseignement Supérieur et de la Recherche Scientifique and the Ministère de Eaux et Fôrests in Côte d’Ivoire, and the Office Ivoirien des Parcs et Réserves for permitting the study in Cote d'Ivoire, Uganda Wildlife Authority and Ugandan National Council for Science and Technology for permitting the study in Uganda, and the staff of the Tai Chimpanzee Project and Budongo Conservation Field Station for their commitment.
Special thanks to Caroline Asiimwe, Pawel Fedurek, Fabian Leendertz and Klaus Zuberbühler for their support.
References
1. Rilling JK, Glasser MF, Preuss TM, et al. The evolution of the arcuate fasciculus revealed with comparative DTI. Nat. Neurosci. 2008;11:426–428.
2. Ardesch DJ, Scholtens LH, Li L, Preuss TM, Rilling JK, van den Heuvel MP. Evolutionary expansion of connectivity between multimodal association areas in the human brain compared with chimpanzees. Proc. Natl. Acad. Sci. U. S. A. 2019;116:7101–7106.
3. Cohen J. Animal studies. NIH to end chimp breeding for research. Science 2007;316:1265.
4. Roebroeck A, Miller KL, Aggarwal M. Ex vivo diffusion MRI of the human brain: Technical challenges and recent advances. NMR Biomed. 2019;32:e3941.
5. Heuer K, Gulban OF, Bazin P-L, et al. Evolution of neocortical folding: A phylogenetic comparative analysis of MRI from 34 primate species. Cortex 2019;118:275–291.
6. Reese TG, Heid O, Weisskoff RM, Wedeen VJ. Reduction of eddy-current-induced distortion in diffusion MRI using a twice-refocused spin echo. Magn. Reson. Med. 2003;49:177–182.
7. Eichner C, Paquette M, Mildner T, et al. Increased sensitivity and signal-to-noise ratio in diffusion-weighted MRI using multi-echo acquisitions. Neuroimage 2020;221:117172.
8. Skare S. Balchandani P. Newbould R. D. Bammer R. Adiabatic refocusing pulses in 3T and 7T diffusion imaging. In: Proc. Intl. Soc. Mag. Reson. Med. 15 (2007). ; 2015.
9. Yang QX, Posse S, Le Bihan D, Smith MB. Double-sampled echo-planar imaging at 3 tesla. J. Magn. Reson. B 1996;113:145–150.
10. Andersson JLR, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage 2003;20:870–888.
11. Gudbjartsson H, Patz S. The Rician distribution of noisy MRI data. Magn. Reson. Med. 1995;34:910–914.
12. Cordero-Grande L, Christiaens D, Hutter J, Price AN, Hajnal JV. Complex diffusion-weighted image estimation via matrix recovery under general noise models. Neuroimage 2019;200:391–404.
13. Andersson JLR, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage 2016;125:1063–1078.
Figures