0886
Whole-brain fMRI at 5 frames per second using T-Hex spiral acquisition1Institute for Biomedical Engineering, ETH Zurich, Zurich, Switzerland
Synopsis
In this work we show high-temporal resolution fMRI using T-Hex spiral-in trajectories. 3mm-resolved whole-brain volumes are acquired at a frame rate of 5Hz.
Introduction
Rapid whole-brain MRI with acquisition rates of several frames per second is often aimed at in BOLD fMRI. It is necessary for studying functional connectivity of different brain regions (1–3), helps removing physiological confounds (4), facilitates retrospective head motion correction and enables location-dependent measurement of the haemodynamic response function. One means of accomplishing whole-brain MRI in fractions of a second is MR-encephalography (5–7). However, strongly relying on coil sensitivity encoding, the spatial resolution of these techniques diminishes with depth in the brain, dropping to 2-3cm. Conventional multiband or 3D EPI sampling strategies on the other hand only offer TRs down to roughly 500ms for 3mm resolved isotropic whole-brain coverage (1). A promising recent alternative is T-Hex encoding with spiral readouts (8), which combines uniform resolution with near-optimal undersampling and exploitation of gradient capabilities. Here, we explore the potential of this approach to speed up whole-brain fMRI. It is shown to enable scans of 3mm resolution in less than 200ms. Voxel-wise signal spectra illustrate that this frame-rate suffices to eliminate contamination by cardiovascular dynamics including 2nd harmonics.Methods
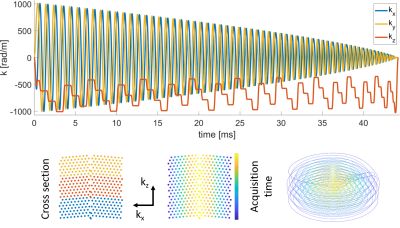
Scanning was performed under local ethics approval on a 3T Philips Achieva scanner using a 16-channel receive coil array with 16 integrated field probes (9) (NeuroCam, Skope MR Technologies, Zurich, Switzerland). Two in-vivo fMRI experiments were conducted using visually stimulated, simple tapping paradigms, involving both hands (A) /the right hand (B). Each interval being 31.5ms/28.3ms long. For the imaging part, T-Hex spiral-in trajectories (generating lattice vectors v = [6,1] / v = [4,3] ) as described in (8) were embedded in gradient echo sequences (Figure 1). Three shots of 50ms/44ms acquisition duration each, resolved the entire brain (FOV = 24 x 24 x 12 cm3) isotropically with 3.1mm and an undersampling factor of R = 7/8. TE = 52.5ms/46ms, TRvolume = 196.56ms/177ms, 358/1872 dynamics. Images were reconstructed using an iterative cg-SENSE reconstruction(8,10), including multi-frequency-interpolation(11,12) for static off-resonance correction in each iteration, and the concurrently monitored encoding dynamics up to 3rd order (13,14). Off-resonance and coil sensitivity maps were derived from a 3D multi-echo, spin-warp pre-scan (6 echoes, TE 2-7 ms, 1.5x1.5x1.5mm3 resolution). The voxel-wise time series of the magnitude data were analyzed using a general linear model (GLM) (15) (SPM12, http://www.fil.ion.ucl.ac.uk/spm/software/spm12/). The GLM was based on a block design and additionally included six rigid-body motion parameters derived from co-registration of the fMRI images.Results
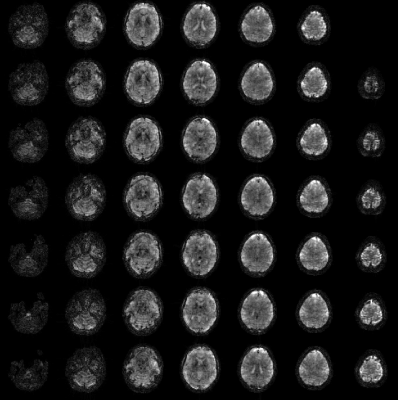
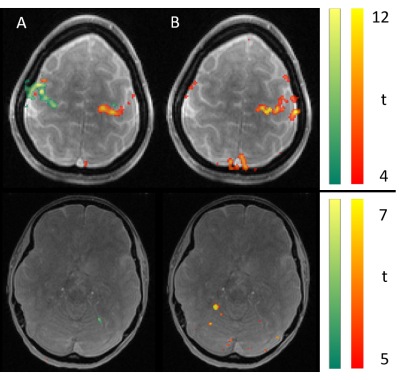
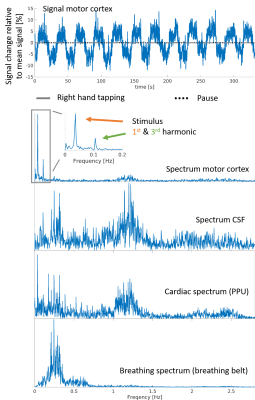
Figure 2 shows the mean images over the entire fMRI run (A). Figure 3 shows the BOLD activation for both runs as overlay on the the anatomic pre-scan. Figure 4 shows time course and spectra of run B: 10 activated voxel together with cardiac and respiratory spectra as they were recorded using a PPU and breathing belt.Discussion
The present work shows fMRI data with an unprecedented spatiotemporal resolution. This is enabled by highly efficient T-Hex spiral sampling. Robust functional activation can be seen in the motor cortex as expected and corroborates that the acquisition efficiency of T-Hex readout schemes can be utilized for and satisfies the requirements of very fast functional scanning. Spectral analysis of voxel-wise dynamics reveals physiological signal, which is in good agreement with the conventional monitoring results. Functional time series imaging purged from physiological confounds without the need for additional measurements will benefit many neuroscientific applications.Acknowledgements
No acknowledgement found.References
1. Feinberg DA, Moeller S, Smith SM, et al. Multiplexed Echo Planar Imaging for Sub-Second Whole Brain FMRI and Fast Diffusion Imaging Valdes-Sosa PA, editor. PLoS ONE 2010;5:e15710 doi: 10.1371/journal.pone.0015710.
2. Smith SM, Miller KL, Salimi-Khorshidi G, et al. Network modelling methods for FMRI. NeuroImage 2011;54:875–891 doi: 10.1016/j.neuroimage.2010.08.063.
3. Frässle S, Lomakina EI, Razi A, Friston KJ, Buhmann JM, Stephan KE. Regression DCM for fMRI. NeuroImage 2017;155:406–421 doi: 10.1016/j.neuroimage.2017.02.090.
4. Huotari N, Raitamaa L, Helakari H, et al. Sampling Rate Effects on Resting State fMRI Metrics. Front. Neurosci. 2019;13 doi: 10.3389/fnins.2019.00279.
5. Lin F-H, Wald LL, Ahlfors SP, Hämäläinen MS, Kwong KK, Belliveau JW. Dynamic magnetic resonance inverse imaging of human brain function. Magn Reson Med 2006;56:787–802 doi: 10.1002/mrm.20997.
6. Lin F-H, Witzel T, Mandeville JB, et al. Event-related single-shot volumetric functional magnetic resonance inverse imaging of visual processing. NeuroImage 2008;42:230–247 doi: 10.1016/j.neuroimage.2008.04.179.
7. Hennig J, Zhong K, Speck O. MR-Encephalography: Fast multi-channel monitoring of brain physiology with magnetic resonance. NeuroImage 2007;34:212–219 doi: 10.1016/j.neuroimage.2006.08.036.
8. Engel M, Kasper L, Wilm B, et al. T-Hex: Tilted hexagonal grids for rapid 3D imaging. Magnetic Resonance in Medicine n/a doi: https://doi.org/10.1002/mrm.28600.
9. Brunner DO, Gross S, Schmid T, et al. Integration of field monitoring for neuroscientific applications - SNR, acceleration and image integrity. In: Proceedings of the 27th Annual Meeting of ISMRM. Montréal, Canada; 2019. p. 1046.
10. Pruessmann KP, Weiger M, Börnert P, Boesiger P. Advances in sensitivity encoding with arbitrary k-space trajectories. Magn. Reson. Med. 2001;46:638–651 doi: 10.1002/mrm.1241.
11. Man L-C, Pauly JM, Macovski A. Multifrequency interpolation for fast off-resonance correction. Magn. Reson. Med. 1997;37:785–792 doi: 10.1002/mrm.1910370523.
12. Barmet C, Tsao J, Pruessmann KP. Sensitivity encoding and B0 inhomogeneity – A simultaneous reconstruction approach. In: Proceedings of the 13th Annual Meeting of ISMRM. Miami, Florida; 2005. p. 682.
13. Barmet C, Wilm BJ, Pavan M, et al. Concurrent higher-order field monitoring for routine head MRI: an integrated heteronuclear setup. In: Proceedings of the 18th Annual Meeting of ISMRM. Stockholm, Sweden; 2010. p. 216.
14. Wilm BJ, Barmet C, Pavan M, Pruessmann KP. Higher order reconstruction for MRI in the presence of spatiotemporal field perturbations. Magn. Reson. Med. 2011;65:1690–1701 doi: 10.1002/mrm.22767.
15. Friston KJ, Jezzard P, Turner R. Analysis of functional MRI time-series. Human Brain Mapping 1994;1:153–171 doi: 10.1002/hbm.460010207.
Figures