0622
Frequency Drift in MR Spectroscopy: An 87-scanner 3T Phantom Study
Steve C.N. Hui1,2, Mark Mikkelsen1,2, Helge J. Zöllner1,2, Vishwadeep Ahluwalia3, Sarael Alcauter4, Laima Baltusis5, Deborah A. Barany6, Laura R. Barlow7, Robert Becker8, Jeffrey I. Berman9, Adam Berrington10, Pallab K. Bhattacharyya11, Jakob Udby Blicher12, Wolfgang Bogner13, Mark S. Brown14, Vince D. Calhoun15, Ryan Castillo16, Kim M. Cecil17, Yeo Bi Choi18, Winnie C.W. Chu19, William T. Clarke20, Alexander R. Craven21, Koen Cuypers22, Michael Dacko23, Camilo de la Fuente-Sandoval24, Patricia Desmond25, Aleksandra Domagalik26, Julien Dumont27, Niall W. Duncan28, Ulrike Dydak29, Katherine Dyke30, David A. Edmondson17, Gabriele Ende8, Lars Ersland31, C. John Evans32, Alan S. R. Fermin33, Antonio Ferretti34, Ariane Fillmer35, Tao Gong36, Ian Greenhouse37, James T. Grist38, Meng Gu39, Ashley D. Harris40, Katarzyna Hat41, Stefanie Heba42, Eva Heckova13, John P. Hegarty II43, Kirstin-Friederike Heise44, Aaron Jacobson45, Jacobus F.A. Jansen46, Christopher W. Jenkins47, Stephen J. Johnston48, Christoph Juchem49, Alayar Kangarlu50, Adam B. Kerr5, Karl Landheer51, Thomas Lange52, Phil Lee53, Swati Rane Levendovszky54, Catherine Limperopoulos55, Feng Liu56, William Lloyd57, David J. Lythgoe58, Maro G. Machizawa59, Erin L. MacMillan7, Richard J. Maddock60, Andrei V. Manzhurtsev61, María L. Martinez-Gudino62, Jack J. Miller63, Heline Mirzakhanian64, Paul G. Mullins65, Jamie Near66, Wibeke Nordhøy67, Georg Oeltzschner1,2, Raul Osorio62, Maria C.G. Otaduy68, Erick H. Pasaye4, Ronald Peeters69, Scott J. Peltier70, Ulrich Pilatus71, Nenad Polomac71, Eric C. Porges72, Subechhya Pradhan55, James Joseph Prisciandaro73, Nick Puts74, Caroline D. Rae75, Francisco Reyes-Madrigal76, Timothy P.L. Roberts9, Caroline E. Robertson77, Muhammad G. Saleh78, Jens T. Rosenberg79, Diana-Georgiana Rotaru58, O'Gorman Tuura L. Ruth80, Kristian Sandberg12, Ryan Sangill81, Keith Schembri82, Anouk Schrantee83, Natalia A. Semenova84, Debra Singel85, Rouslan Sitnikov86, Jolinda Smith87, Yulu Song36, Craig Stark88, Diederick Stoffers89, Stephan P. Swinnen44, Costin Tanase60, Sofie Tapper1,2, Martin Tegenthoff42, Thomas Thiel90, Marc Thioux91, Peter Truong92, Pim van Dijk91, Nolan Vella82, Rishma Vidyasagar93, Andrej Vovk94, Guangbin Wang36, Lars T. Westle67, Timothy K. Wilbur54, William R. Willoughby95, Martin Wilson96, Hans-Jörg Wittsack97, Adam J. Woods98, Yen-Chien Wu99, Junqian Xu100, Maria Yanez Lopez101, David K.W. Yeung19, Qun Zhao102, Xiaopeng Zhou29, Gasper Zupan94, and Richard A.E. Edden1,2
1Russell H. Morgan Department of Radiology and Radiological Science, The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 2F.M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States, 3GSU/GT Center for Advanced Brain Imaging, Georgia Institute of Technology, Atlanta, GA, United States, 4Instituto de Neurobiología, Universidad Nacional Autónoma de México, Queretaro, Mexico, 5Center for Cognitive and Neurobiological Imaging, Stanford University, Stanford, CA, United States, 6Kinesiology, University of Georgia, Athens, GA, United States, 7Department of Radiology, The University of British Columbia, Vancouver, BC, Canada, 8Center for Innovative Psychiatry and Psychotherpay Research, Department Neuroimaging, Central Institute of Mental Health, Medical Faculty Mannheim, Heidelberg University, Mannheim, Germany, 9Department of Radiology, Children's Hospital of Philadelphia, Philadelphia, PA, United States, 10Sir Peter Mansfield Imaging Centre, School of Physics and Astronomy, University of Nottingham, Nottingham, United Kingdom, 11Imaging Institute, The Cleveland Clinic, Cleveland, OH, United States, 12Center of Functionally Integrative Neuroscience, Aarhus University, Aarhus, Denmark, 13Department of Biomedical Imaging and Image-guided Therapy, High-Field MR Center, Medical University of Vienna, Vienna, Austria, 14Department of Radiology, University of Colorado Anschutz Medical Campus, Aurora, CO, United States, 15Tri-Institutional Center for Translational Research in Neuroimaging and Data Science(TReNDS), Georgia State University, Georgia Institute of Technology, and Emory University, Atlanta, GA, United States, 16Neuroscience Research AustraliaNeuRA Imaging, Randwick, Australia, 17Department of Radiology, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States, 18Psychological and Brain Sciences, Dartmouth College, Hanover, NH, United States, 19Department of Imaging & Interventional Radiology, The Chinese University of Hong Kong, Hong Kong, Hong Kong, 20Wellcome Centre for Integrative Neuroimaging, NDCN, University of Oxford, Oxford, United Kingdom, 21Department of Biological and Medical Psychology, University of Bergen, Haukeland University Hospital, Bergen, Norway, 22REVAL Rehabilitation Research Institute (REVAL), Hasselt University, Diepenbeek, Belgium, 23Department of Radiology, Medical Physics, Medical Center - University of Freiburg, Faculty of Medicine, University of Freiburg, Freiburg, Germany, 24Laboratory of Experimental Psychiatry & Neuropsychiatry Department, Instituto Nacional de Neurología y Neurocirugía, Mexico City, Mexico, 25Department of Radiology, University of Melbourne/ Royal Melbourne Hospital, Melbourne, Australia, 26Malopolska Centre of Biotechnology, Jagiellonian University, Krakow, Poland, 27Clinical Imaging Core Facility, CI2C Lille, Lille, France, 28Graduate Institute of Mind, Brain and Consciousness, Taipei Medical University, Taipei, Taiwan, 29School of Health Sciences, Purdue University, West Lafayette, IN, United States, 30School of Psychology, University of Nottingham, Nottingham, United Kingdom, 31Department of Clinical Engineering, University of Bergen, Haukeland University Hospital, Bergen, Norway, 32CUBRIC, Cardiff University, Cardiff, United Kingdom, 33Center for Brain, Mind and Kansei Sciences Research, Hiroshima University, Hiroshima, Japan, 34Neuroscience, Imaging and Clinical Sciences, University "G. d'Annunzio" of Chieti-Pescara, Chieti, Italy, 35Physikalisch-Technische Bundesanstalt (PTB), Braunschweig und Berlin, Germany, 36Department of Imaging and Nuclear Medicine, Shandong Medical Imaging Research Institute, Shandong University, Jinan, China, 37Human Physiology, University of Oregon, Eugene, OR, United States, 38Physiology, Anatomy, and Genetics/ Oxford Centre for Magnetic Resonance, The University of Oxford / Department of Radiology, The Churchill Hospital, The University of Oxford, Oxford, United Kingdom, 39Department of Radiology, Stanford University, Stanford, CA, United States, 40Department of Radiology, University of Calgary, Calgary, AB, Canada, 41Institute of Psychology, Jagiellonian University, Krakow, Poland, 42Department of Neurology, BG University Hospital Bergmannsheil, Bochum, Germany, 43Psychiary & Behavioral Sciences, Stanford University, Stanford, CA, United States, 44Department of Movement Sciences, KU Leuven, Leuven, Belgium, 45Department of Radiology, University of California San Diego, San Diego, CA, United States, 46Department of Radiology and Nuclear Medicine, Maastricht University Medical Center, Maastricht, Netherlands, 47CUBRIC, Cardiff university, Cardiff, United Kingdom, 48Psychology Dept. / Clinical Imaging Facility, Swansea University, Swansea, United Kingdom, 49Biomedical Engineering and Radiology, Columbia University, New York City, NY, United States, 50Psychiatry, Columbia University Irving Medical Center/New York State Psychiatric Institute, New York City, NY, United States, 51Biomedical Engineering, Columbia University, New York City, NY, United States, 52Department of Radiology, Medical Physics, University of Freiburg, Freiburg, Germany, 53Department of Radiology, University of Kansas Medical Center, Kansas, KS, United States, 54Department of Radiology, University of Washington, Seattle, WA, United States, 55Developing Brain Institute, Diagnostic Imaging and Radiology, Children's National Hospital, Washington, DC, United States, 56Department of Psychiatry, Columbia University Irving Medical Center/New York State Psychiatric Institute, New York, NY, United States, 57Division of Informatics, Imaging & Data Sciences, University of Manchester, Manchester, United Kingdom, 58Department of Neuroimaging, King's College London, London, United Kingdom, 59Center for Brain, Mind and KANSEI Sciences Research, Hiroshima University, Hiroshima, Japan, 60Psychiatry and Behavioral Sciences, University of California Davis, Imaging Research Center, Davis, CA, United States, 61Department of Radiology, Clinical and Research Institute of Emergency Pediatric Surgery and Trauma, Moscow, Russian Federation, 62Imágenes Cerebrales, Instituto Nacional de Psiquiatría Ramón de la Fuente, Mexico City, Mexico, 63Department of Physics, University of Oxford, Oxford, United Kingdom, 64Department of Psychiatry, University of California San Diego, San Diego, CA, United States, 65Department of Psychology, Bangor University, Bangor, United Kingdom, 66Douglas Mental Health University Institute and Department of Psychiatry, McGill University, Montreal, QC, Canada, 67Department of Diagnostic Physics, Division of Radiology and Nuclear Medicine, Oslo University Hospital, Oslo, Norway, 68LIM44, Instituto e Departamento de Radiologia, Faculdade de Medicina, Universidade de Sao Paulo, Sao Paulo, Brazil, 69Department of Imaging & Pathology, Department of Radiology, University Hospitals Leuven, KU Leuven, Leuven, Belgium, 70Functional MRI Laboratory, University of Michigan, Ann Arbor, MI, United States, 71Institute of Neuroradiology, Goethe-University Frankfurt, Frankfurt, Germany, 72Center for Cognitive Aging and Memory, McKnight Brain Institute, Department of Clinical and Health Psychology, College of Public Health and Health Professions, University of Florida, Gainesville, FL, United States, 73Psychiatry and Behavioral Sciences, Medical University of South Carolina, Charleston, SC, United States, 74Forensic & Neurodevelopmental Sciences, King's College London, London, United Kingdom, 75NeuRA Imaging, Neuroscience Research Australia, Randwick, Australia, 76Laboratory of Experimental Psychiatry, Instituto Nacional de Neurología y Neurocirugía, Mexico City, Mexico, 77Department of Psychological and Brain Sciences, Dartmouth College, Hanover, NH, United States, 78Department of Diagnostic Radiology and Nuclear Medicine, University of Maryland School of Medicine, Baltimore, MD, United States, 79McKnight Brain Institute, AMRIS, University of Florida, Gainesville, FL, United States, 80Center for MR Research, University Children's Hospital, Zurich, Switzerland, 81Center of Functionally Integrative Neuroscience, Aarhus University Hospital, Aarhus, Denmark, 82Medical Physics, Mater Dei Hospital, Imsida, Malta, 83Department of Radiology and Nuclear Medicine, Amsterdam University Medical Center, University of Amsterdam, Amsterdam, Netherlands, 84504, Emanuel Institute of Biochemical Physics of the Russian Academy of Sciences, Moscow, Russian Federation, 85Psychiatry, University of Colorado Anschutz Medical Campus, Aurora, CO, United States, 86Clinical Neuroscience, MRI Centre, Karolinska Institutet, Clinical Neuroscience, MRI Centre, Sweden, 87Lewis Center for Neuroimaging, University of Oregon, Eugene, OR, United States, 88Department of Neurobiology and Behavior, Facility for Imaging and Brain Research (FIBRE) & Campus Center for Neuroimaging (CCNI), University of California, Irvine, Irvine, CA, United States, 89Spinoza Centre for Neuroimaging, Royal Netherlands Academy of Arts and Sciences, Amsterdam, Netherlands, 90Institute of Clinical Neuroscience and Medical Psychology, University Dusseldorf, Medical Faculty, Düsseldorf, Germany, 91Otorhinolaryngology, Head and Neck Surgery, University of Groningen, University Medical Center Groningen, Groningen, Netherlands, 92Brain Health Imaging Centre, Centre for Addiction and Mental Health, Toronto, ON, Canada, 93Melbourne Dementia Research Centre, Florey Institute of Neurosciences and Mental Health, Melbourne, Australia, 94Faculty of Medicine, University of Ljubljana, Ljubljana, Slovenia, 95Department of Radiology, University of Alabama at Birmingham, Birmingham, AL, United States, 96Centre for Human Brain Health, University of Birmingham, Birmingham, United Kingdom, 97Department of Diagnostic and Interventional Radiology, University Dusseldorf, Medical Faculty, Düsseldorf, Germany, 98Center for Cognitive Aging and Memory, McKnight Brain Institute, Department of Clinical and Health Psychology, College of Public Health and Health Professions. Department of Neuroscience, College of Medicine, University of Florida, Gainesville, FL, United States, 99Department of Radiology, TMU-Shuang Ho Hospital, New Taipei City, Taiwan, 100Department of Radiology and Psychiatry, Baylor College of Medicine, Houston, TX, United States, 101Perinatal Imaging & Health, King's College London, London, United Kingdom, 102Bioimaging Research Center, University of Georgia, Athens, GA, United States
1Russell H. Morgan Department of Radiology and Radiological Science, The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 2F.M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States, 3GSU/GT Center for Advanced Brain Imaging, Georgia Institute of Technology, Atlanta, GA, United States, 4Instituto de Neurobiología, Universidad Nacional Autónoma de México, Queretaro, Mexico, 5Center for Cognitive and Neurobiological Imaging, Stanford University, Stanford, CA, United States, 6Kinesiology, University of Georgia, Athens, GA, United States, 7Department of Radiology, The University of British Columbia, Vancouver, BC, Canada, 8Center for Innovative Psychiatry and Psychotherpay Research, Department Neuroimaging, Central Institute of Mental Health, Medical Faculty Mannheim, Heidelberg University, Mannheim, Germany, 9Department of Radiology, Children's Hospital of Philadelphia, Philadelphia, PA, United States, 10Sir Peter Mansfield Imaging Centre, School of Physics and Astronomy, University of Nottingham, Nottingham, United Kingdom, 11Imaging Institute, The Cleveland Clinic, Cleveland, OH, United States, 12Center of Functionally Integrative Neuroscience, Aarhus University, Aarhus, Denmark, 13Department of Biomedical Imaging and Image-guided Therapy, High-Field MR Center, Medical University of Vienna, Vienna, Austria, 14Department of Radiology, University of Colorado Anschutz Medical Campus, Aurora, CO, United States, 15Tri-Institutional Center for Translational Research in Neuroimaging and Data Science(TReNDS), Georgia State University, Georgia Institute of Technology, and Emory University, Atlanta, GA, United States, 16Neuroscience Research AustraliaNeuRA Imaging, Randwick, Australia, 17Department of Radiology, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States, 18Psychological and Brain Sciences, Dartmouth College, Hanover, NH, United States, 19Department of Imaging & Interventional Radiology, The Chinese University of Hong Kong, Hong Kong, Hong Kong, 20Wellcome Centre for Integrative Neuroimaging, NDCN, University of Oxford, Oxford, United Kingdom, 21Department of Biological and Medical Psychology, University of Bergen, Haukeland University Hospital, Bergen, Norway, 22REVAL Rehabilitation Research Institute (REVAL), Hasselt University, Diepenbeek, Belgium, 23Department of Radiology, Medical Physics, Medical Center - University of Freiburg, Faculty of Medicine, University of Freiburg, Freiburg, Germany, 24Laboratory of Experimental Psychiatry & Neuropsychiatry Department, Instituto Nacional de Neurología y Neurocirugía, Mexico City, Mexico, 25Department of Radiology, University of Melbourne/ Royal Melbourne Hospital, Melbourne, Australia, 26Malopolska Centre of Biotechnology, Jagiellonian University, Krakow, Poland, 27Clinical Imaging Core Facility, CI2C Lille, Lille, France, 28Graduate Institute of Mind, Brain and Consciousness, Taipei Medical University, Taipei, Taiwan, 29School of Health Sciences, Purdue University, West Lafayette, IN, United States, 30School of Psychology, University of Nottingham, Nottingham, United Kingdom, 31Department of Clinical Engineering, University of Bergen, Haukeland University Hospital, Bergen, Norway, 32CUBRIC, Cardiff University, Cardiff, United Kingdom, 33Center for Brain, Mind and Kansei Sciences Research, Hiroshima University, Hiroshima, Japan, 34Neuroscience, Imaging and Clinical Sciences, University "G. d'Annunzio" of Chieti-Pescara, Chieti, Italy, 35Physikalisch-Technische Bundesanstalt (PTB), Braunschweig und Berlin, Germany, 36Department of Imaging and Nuclear Medicine, Shandong Medical Imaging Research Institute, Shandong University, Jinan, China, 37Human Physiology, University of Oregon, Eugene, OR, United States, 38Physiology, Anatomy, and Genetics/ Oxford Centre for Magnetic Resonance, The University of Oxford / Department of Radiology, The Churchill Hospital, The University of Oxford, Oxford, United Kingdom, 39Department of Radiology, Stanford University, Stanford, CA, United States, 40Department of Radiology, University of Calgary, Calgary, AB, Canada, 41Institute of Psychology, Jagiellonian University, Krakow, Poland, 42Department of Neurology, BG University Hospital Bergmannsheil, Bochum, Germany, 43Psychiary & Behavioral Sciences, Stanford University, Stanford, CA, United States, 44Department of Movement Sciences, KU Leuven, Leuven, Belgium, 45Department of Radiology, University of California San Diego, San Diego, CA, United States, 46Department of Radiology and Nuclear Medicine, Maastricht University Medical Center, Maastricht, Netherlands, 47CUBRIC, Cardiff university, Cardiff, United Kingdom, 48Psychology Dept. / Clinical Imaging Facility, Swansea University, Swansea, United Kingdom, 49Biomedical Engineering and Radiology, Columbia University, New York City, NY, United States, 50Psychiatry, Columbia University Irving Medical Center/New York State Psychiatric Institute, New York City, NY, United States, 51Biomedical Engineering, Columbia University, New York City, NY, United States, 52Department of Radiology, Medical Physics, University of Freiburg, Freiburg, Germany, 53Department of Radiology, University of Kansas Medical Center, Kansas, KS, United States, 54Department of Radiology, University of Washington, Seattle, WA, United States, 55Developing Brain Institute, Diagnostic Imaging and Radiology, Children's National Hospital, Washington, DC, United States, 56Department of Psychiatry, Columbia University Irving Medical Center/New York State Psychiatric Institute, New York, NY, United States, 57Division of Informatics, Imaging & Data Sciences, University of Manchester, Manchester, United Kingdom, 58Department of Neuroimaging, King's College London, London, United Kingdom, 59Center for Brain, Mind and KANSEI Sciences Research, Hiroshima University, Hiroshima, Japan, 60Psychiatry and Behavioral Sciences, University of California Davis, Imaging Research Center, Davis, CA, United States, 61Department of Radiology, Clinical and Research Institute of Emergency Pediatric Surgery and Trauma, Moscow, Russian Federation, 62Imágenes Cerebrales, Instituto Nacional de Psiquiatría Ramón de la Fuente, Mexico City, Mexico, 63Department of Physics, University of Oxford, Oxford, United Kingdom, 64Department of Psychiatry, University of California San Diego, San Diego, CA, United States, 65Department of Psychology, Bangor University, Bangor, United Kingdom, 66Douglas Mental Health University Institute and Department of Psychiatry, McGill University, Montreal, QC, Canada, 67Department of Diagnostic Physics, Division of Radiology and Nuclear Medicine, Oslo University Hospital, Oslo, Norway, 68LIM44, Instituto e Departamento de Radiologia, Faculdade de Medicina, Universidade de Sao Paulo, Sao Paulo, Brazil, 69Department of Imaging & Pathology, Department of Radiology, University Hospitals Leuven, KU Leuven, Leuven, Belgium, 70Functional MRI Laboratory, University of Michigan, Ann Arbor, MI, United States, 71Institute of Neuroradiology, Goethe-University Frankfurt, Frankfurt, Germany, 72Center for Cognitive Aging and Memory, McKnight Brain Institute, Department of Clinical and Health Psychology, College of Public Health and Health Professions, University of Florida, Gainesville, FL, United States, 73Psychiatry and Behavioral Sciences, Medical University of South Carolina, Charleston, SC, United States, 74Forensic & Neurodevelopmental Sciences, King's College London, London, United Kingdom, 75NeuRA Imaging, Neuroscience Research Australia, Randwick, Australia, 76Laboratory of Experimental Psychiatry, Instituto Nacional de Neurología y Neurocirugía, Mexico City, Mexico, 77Department of Psychological and Brain Sciences, Dartmouth College, Hanover, NH, United States, 78Department of Diagnostic Radiology and Nuclear Medicine, University of Maryland School of Medicine, Baltimore, MD, United States, 79McKnight Brain Institute, AMRIS, University of Florida, Gainesville, FL, United States, 80Center for MR Research, University Children's Hospital, Zurich, Switzerland, 81Center of Functionally Integrative Neuroscience, Aarhus University Hospital, Aarhus, Denmark, 82Medical Physics, Mater Dei Hospital, Imsida, Malta, 83Department of Radiology and Nuclear Medicine, Amsterdam University Medical Center, University of Amsterdam, Amsterdam, Netherlands, 84504, Emanuel Institute of Biochemical Physics of the Russian Academy of Sciences, Moscow, Russian Federation, 85Psychiatry, University of Colorado Anschutz Medical Campus, Aurora, CO, United States, 86Clinical Neuroscience, MRI Centre, Karolinska Institutet, Clinical Neuroscience, MRI Centre, Sweden, 87Lewis Center for Neuroimaging, University of Oregon, Eugene, OR, United States, 88Department of Neurobiology and Behavior, Facility for Imaging and Brain Research (FIBRE) & Campus Center for Neuroimaging (CCNI), University of California, Irvine, Irvine, CA, United States, 89Spinoza Centre for Neuroimaging, Royal Netherlands Academy of Arts and Sciences, Amsterdam, Netherlands, 90Institute of Clinical Neuroscience and Medical Psychology, University Dusseldorf, Medical Faculty, Düsseldorf, Germany, 91Otorhinolaryngology, Head and Neck Surgery, University of Groningen, University Medical Center Groningen, Groningen, Netherlands, 92Brain Health Imaging Centre, Centre for Addiction and Mental Health, Toronto, ON, Canada, 93Melbourne Dementia Research Centre, Florey Institute of Neurosciences and Mental Health, Melbourne, Australia, 94Faculty of Medicine, University of Ljubljana, Ljubljana, Slovenia, 95Department of Radiology, University of Alabama at Birmingham, Birmingham, AL, United States, 96Centre for Human Brain Health, University of Birmingham, Birmingham, United Kingdom, 97Department of Diagnostic and Interventional Radiology, University Dusseldorf, Medical Faculty, Düsseldorf, Germany, 98Center for Cognitive Aging and Memory, McKnight Brain Institute, Department of Clinical and Health Psychology, College of Public Health and Health Professions. Department of Neuroscience, College of Medicine, University of Florida, Gainesville, FL, United States, 99Department of Radiology, TMU-Shuang Ho Hospital, New Taipei City, Taiwan, 100Department of Radiology and Psychiatry, Baylor College of Medicine, Houston, TX, United States, 101Perinatal Imaging & Health, King's College London, London, United Kingdom, 102Bioimaging Research Center, University of Georgia, Athens, GA, United States
Synopsis
This project aimed to examine the relationship between gradient-induced heating and field drift on a large sample of MRI scanners. A standardized phantom protocol was established, and spectroscopy was performed before and after running 10 minutes of echo-planar imaging (EPI). MRS data were acquired from 87 scanners. The frequency drift trace was extracted by modeling the water signal in each transient. Drift rates of up to 1.3 Hz/minute were seen before EPI, and 4 Hz/minute after. This dataset will allow sites to benchmark scanner drift, for consideration in planning research protocol order and examine the need for real-time field-frequency locking.
Introduction
Heating of the gradient coils and thermal dissipation to the passive shims is a common cause of instability in the B0 field, especially when echo-planar imaging (EPI) sequences are used (1-3). B0 field drift changes the resonance frequency of spins, resulting in line broadening, decreased SNR and changes in editing efficiency for edited MRS experiments which rely on accurately placing frequency-selective pulses. To examine the extent and impact of gradient-induced frequency drift, a standardized protocol was distributed to sites with scanners from three vendors. By collecting data from a large number of sites, we aim to establish ‘typical’ levels of drift for benchmarking purposes, and to assess whether there is a widespread need for real-time field-frequency locking (2).Methods
Phantom water signals were acquired with PRESS localization before and after a BOLD-weighted fMRI sequence. Standardized protocols were generated for GE, Philips and Siemens scanners consisting of: minimal preparatory imaging; pre-fMRI PRESS (TR/TE 5000/35 ms; 64 transients with data stored separately; no water suppression; voxel size 2 × 2 × 2 cm3, duration = 5:20 min); EPI BOLD sequence based on the ADNI-3 (4) protocol (TR/TE 3000/30 ms; 197 dynamics of one average; EPI factor 31, scan duration 10 min); and a long post-fMRI PRESS sequence (360 transients; other parameters same as pre-fMRI PRESS). Sites were instructed to use a water-dominant phantom of spherical or cylindrical shape. Phased-array head or head-and-neck coils with between 8 and 64 channels were used. Scanning was performed at least 6 hours after the previous scan to avoid any drift confounds due to heating effects. Phantoms were acclimatized in the scan room for the same period, and positioned at scanner isocenter. Spectral analysis was performed using MATLAB (R2020b, MathWorks, Natick, USA), including eddy-current correction, zero-filling and Fourier transformation. Frequency-domain pre- and post-fMRI PRESS spectra were modeled using a Lorentzian-Gaussian (Voigt) lineshape model to extract the water peak center frequency from each individual transient. In order to compare frequency drift before and after EPI, the mean absolute frequency offset of the first 64 dynamics was calculated, and paired t-test was performed between pre- and post-fMRI PRESS. To visualize the effect of the observed frequency drifts on a typical 5-minute in vivo protocol, a simulated in vivo spectrum was generated using FID-A (5), including 18 major metabolites (TE = 35 ms; 2048 samples; 2 kHz spectral width, 2 Hz linewidth). The simulated spectrum was convolved with the frequency trace from the first 64 TRs of the phantom recording and spectra plotted. Fifty-eight sites repeated the acquisition protocol on a different day to allow investigation of the reproducibility of frequency drift traces. Pearson and intraclass correlation coefficients (ICC) were calculated between the two runs using the ‘psych’ R package (6). ICC calculation was based on a two-way mixed-effects model with average measures of absolute agreement.Results
Data were received from 71 sites and 87 scanners (GE = 20, Philips = 28, Siemens = 39; 58 scanners submitted repeat data). Figure 1 shows the individual spectra for the highest-drifting scanner, before and after fMRI, with the frequency drift traces. Figure 2 shows the frequency drift traces overlaid for all 87 scanners. Scanners drifted by up to 7 Hz within 5 minutes before fMRI and by up to 26 Hz within 30 minutes after fMRI. Figure 3 shows a box plot of the absolute average frequency offset of each scanner (7). The mean absolute frequency offset across 64 transients (~5 min) was 0.78 ± 0.87 Hz (median = 0.4 Hz) and 1.33 ± 1.42 Hz (median = 0.8 Hz) respectively before and after fMRI. T-tests indicated drifting was significantly increased (p < 0.05) after fMRI, as expected. Simulated spectra that have been convolved with 64-transient water traces (the highest and lowest drift case for each vendor pre- and post-fMRI) are shown in Figure 4. The intensity of the NAA singlet is reduced by up to 26%, 44 % and 18% for GE, Philips and Siemens respectively, after fMRI. Since drift does not impact the noise, these peak signal losses represent predicted losses of SNR. Drift behavior was well correlated and reproducible. ICCs were 0.85 and 0.95 for pre- and post-fMRI PRESS repeated datasets, respectively. Pearson correlation coefficients (0.74 and 0.90) also showed good correlation between repeated datasets.Discussion
Frequency drift data are presented for eighty-seven 3T MRI scanners. Median levels of drift were relatively low (5-minute average under 1 Hz), but the most extreme case suffered from higher levels of drift (up to 3.5 Hz before and 7.2 Hz after fMRI). These levels of drift lead to a measurable loss in SNR for short-TE MRS, as well as changes in editing efficiency and subtraction artefacts in edited MRS. Although the difference between pre- and post-fMRI was significant, it was lower than expected and there appears to be substantial drift associated with running scans ‘from cold’, as indicated by the pre-fMRI traces after only minimal preparatory imaging. Correlation analysis indicated that the drift was highly repeatable between sessions, so one might expect drift associated with previous scans within a protocol to be consistent.Acknowledgements
This work was supported by NIH grants R01 EB016089, R01 EB023963, R21 AG060245, K99 EB028828 and K99 AG062230.References
- Foerster BU, Tomasi D, Caparelli EC. Magnetic field shift due to mechanical vibration in functional magnetic resonance imaging. Magn Reson Med 2005;54(5):1261-1267.
- Henry PG, van de Moortele PF, Giacomini E, Nauerth A, Bloch G. Field-frequency locked in vivo proton MRS on a whole-body spectrometer. Magn Reson Med 1999;42(4):636-642.
- Rowland BC, Liao H, Adan F, Mariano L, Irvine J, Lin AP. Correcting for Frequency Drift in Clinical Brain MR Spectroscopy. J Neuroimaging 2017;27(1):23-28.
- Weiner MW, Veitch DP, Aisen PS, et al. The Alzheimer's Disease Neuroimaging Initiative 3: Continued innovation for clinical trial improvement. Alzheimers Dement 2017;13(5):561-571.
- Simpson R, Devenyi GA, Jezzard P, Hennessy TJ, Near J. Advanced processing and simulation of MRS data using the FID appliance (FID-A)-An open source, MATLAB-based toolkit. Magn Reson Med 2017;77(1):23-33.
- R Core Team R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2020.
- Zöllner HJ, Považan M, Hui SCN, Tapper S, Edden RAE, Oeltzschner G. Comparison of different linear-combination modelling algorithms for short-TE proton spectra. bioRxiv 2020:2020.2006.2005.136796.
Figures

Figure 1. Individual transients of pre- and post-fMRI PRESS (plotted in blue and red, respectively) from the highest-drifting scanner. The frequency offset derived from modeling the water signals is plotted (middle). 360 averages correspond to 30 minutes total scan duration.
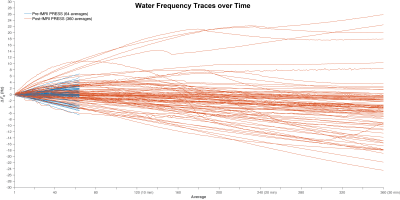
Figure 2. Water offset traces of all 87 scanners. Pre- and post-fMRI traces are plotted in blue and red, respectively. 360 averages correspond to 30 minutes total scan duration.
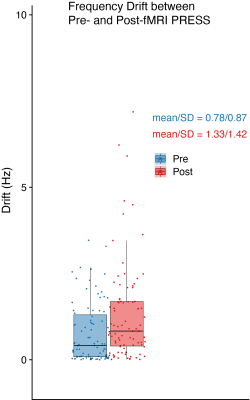
Figure 3. Box plot for the mean absolute frequency offset for pre- and post-fMRI PRESS data on each scanner.
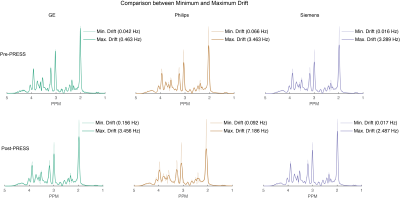
Figure 4. Comparison of simulated spectra with frequency drift applied between minimum and maximum drift for pre- and post-fMRI PRESS data. The minimum-drift case for each vendor (50% opacity) is overlaid with the maximum-drift case (opaque).