0119
Motion-corrected 3D-EPTI with 4D navigator for fast and robust whole-brain quantitative imaging1Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 2Department of Electrical Engineering and Computer Science, MIT, Cambridge, MA, United States, 3Harvard-MIT Health Sciences and Technology, MIT, Cambridge, MA, United States, 4Tsinghua University, Beijing, China, 5Department of Radiology, Stanford University, Stanford, CA, United States, 6Department of Electrical Engineering, Stanford University, Stanford, CA, United States
Synopsis
A motion-correction method is developed for the recently proposed 3D-EPTI to achieve fast and motion-robust quantitative imaging of the human brain. A 4D-navigator (x-y-z-echoes) is inserted into the relaxation-recovery dead-time of the sequence to provide accurate estimations of 3D-motion and B0-inhomogeneity changes at every TR (~2-3s), which are incorporated into a motion-and-phase corrected subspace reconstruction. The navigator utilizes an optimized spatiotemporal encoding to acquire central 3D k-space for accurate motion-estimation using just 4 small-flip-angle excitations, resulting in negligible signal-recovery reduction (<1%) to the 3D-EPTI acquisition. Simulation and in-vivo experiments preliminarily validate the accuracy of estimation and effectiveness of the reconstruction.
Introduction
Quantitative multi-parametric imaging provides more repeatable and direct measurements than conventional weighted imaging, but requires longer scan time which increases their sensitivity to motion. Many emerging techniques have been developed to achieve faster and more robust quantitative measurements, such as MR fingerprinting (MRF)1,2 and multitasking3.Recently, we have developed an efficient quantitative imaging method termed 3D echo-planar time-resolved imaging (3D-EPTI)4,5 that utilizes continuous EPI-based readouts and optimized spatiotemporal encoding to resolve thousands of distortion-free multi-contrast images to track the signal evolution and fit quantitative parameters. Whole-brain T1-T2-T2* maps can be obtained in only 3 minutes at 1-mm isotropic resolution with high repeatability and accuracy. Although this scan time is relatively short, motion during scanning can still affect the image quality and compromise the accuracy of quantitative measurement.
In this work, we develop a new motion correction approach for 3D-EPTI to achieve fast and motion-robust quantitative imaging. A 4D-navigator (x-y-z-echoes) is acquired in each TR during the recovery deadtime to estimate both 3D rigid motion (6 DOF) and B0-inhomogeneity change caused by position change. This navigator utilizes an optimized spatiotemporal encoding to achieve large 3D k-space coverage with just 4 small-flip-angle excitations, resulting in negligible signal reduction (<1%) to the 3D-EPTI acquisition. The estimated motion parameters and B0-inhomogeneity changes are used in the motion-corrected 3D subspace reconstruction. The accuracy of motion estimation and effectiveness of correction are demonstrated by simulation and in-vivo experiments.
Methods
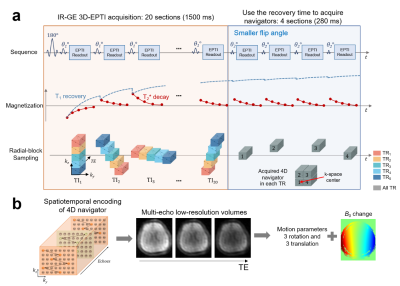
In this study, as a proof-of-concept, the proposed motion-correction approach is applied to the inversion-recovery gradient-echo (IR-GE) 3D-EPTI sequence which can estimate T1 and T2* simultaneously. Figure 1a illustrates the acquisition of IR-GE 3D-EPTI with 4D-navigator, where within a given 3D-EPTI readout block, a large ky-kz-TE region is encoded quickly using an efficient spatiotemporal encoding trajectory6 (Fig.1b-left). For the IR-GE 3D-EPTI, at each inversion time (TI), multiple ky-kz-TE blocks are acquired across different TRs to create a radial-blade sampling. The sharp image at every TE can be resolved from k-t reconstruction resulting in >1000 multi-contrast images for quantitative fitting. For the 4D-navigator (Fig.1a-right), the same 4-block acquisition is performed at each TR, where the 4 blocks are asymmetrically arranged around the center ky-kz position to cover higher frequency signals for more robust motion-estimation. The missing asymmetric signals can be recovered later through subspace reconstruction, and the matrix size of the navigator is 32×24 (ky×kz) after reconstruction with the readout kx direction fully-sampled.Fig. 1b illustrates the processing of the 4D-navigator data. To reconstruct the navigator, the 4 blocks are combined assuming similar contrasts between them, and a low-rank subspace reconstruction6-8 is used to generate a low-resolution image at each echo. Both motion parameters (6 DOF) and B0 inhomogeneity changes are then estimated using this information. The additional B0-inhomogeneity changes, that can be caused by the interaction between the main B0-field and head movements, are estimated here to further improve reconstruction accuracy, since EPTI reconstruction requires the prior knowledge of B0-inhomogeneity to model the phase evolution.
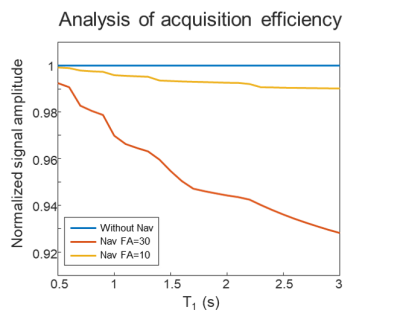
The proposed navigator is acquired during the T1 recovery dead-time of the 3D-EPTI sequence to avoid prolonging the scan. Moreover, smaller flip angles (10° instead of 30° used in the IR-GE 3D-EPTI) is applied to reduce its effect on signal recovery. An EPG-based simulation demonstrates that this resulted in < 1% signal-recovery reduction across the relevant range of T1 values (Fig.2).
A motion-and-phase corrected 3D subspace reconstruction is implemented to obtain the multi-contrast volumes acquired by IR-GE 3D-EPTI using the estimated motion parameters. Quantitative parameters including T1, T2*, PD and B1+ maps are estimated by dictionary matching after reconstruction.
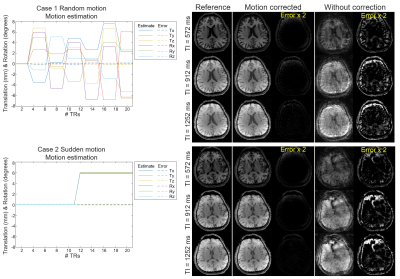
Simulation experiment: motion-corrupted IR-GE 3D-EPTI data with navigators are simulated using previously acquired 3D quantitative maps and EPG model to evaluate motion estimation and correction performance.
In-vivo experiment: IR-GE 3D-EPTI sequence with navigators was used to acquire data on a 3T Prisma scanner using a 32-channel receiver coil. Motion-free IR-GE 3D-EPTI acquisition at 3 different head positions were performed and the data were subsampled and combined together to mimic a motion-corrupted acquisition with head rotation every ~20s. In addition to reconstructing the synthesized motion-corrupted acquisition, full-datasets are reconstructed at the 3 head positions to obtain reference motion parameters, B0-inhomogeneity changes, and quantitative maps. The key acquisition parameters were: FOV = 210×210×150 mm3, 1.5-mm isotropic resolution, TR=2.5s, acquisition time = 75s per dataset.
Results
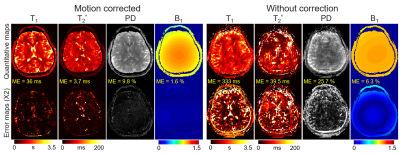
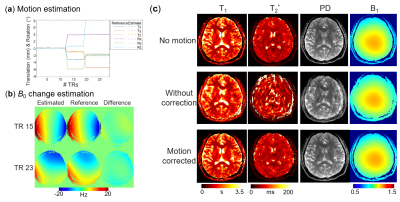
The results of simulation experiment are shown in Fig.3 and 4. In Fig.3, the estimation of motion parameters shows high accuracy in both the random and sudden motion cases, and the motion-corrected subspace reconstruction effectively removes most of the artifacts in the multi-contrast images. Quantitative maps with and without correction are shown in Fig.4, where the corrected maps show significantly improved accuracy when compared to those without correction.Figure 5 shows the results from the in-vivo experiment, where both the estimation of motion parameters and B0-inhomogeneity changes from the 4D-navigators achieve high accuracy with good correspondence to the reference estimations. By incorporating the estimated parameters into the reconstruction, severe motion-induced artifacts in the quantitative maps are markedly reduced.
Conclusion
The proposed motion-corrected 3D-EPTI with 4D-navigator acquisition achieves accurate 3D motion estimation and correction, allowing motion-robust quantitative imaging with negligible cost in scan efficiency.Acknowledgements
This work was supported by the NIH NIBIB (R01-EB020613, R01-EB019437, R01-MH116173, R01EB016695, P41EB030006, and U01-EB025162) and the instrumentation Grants (S10-RR023401, S10-RR023043, and S10-RR019307).References
1. Ma D, Gulani V, Seiberlich N, Liu K, Sunshine JL, Duerk JL, Griswold MA. Magnetic resonance fingerprinting. Nature 2013;495(7440):187-192.
2. Zhao B, Setsompop K, Adalsteinsson E, Gagoski B, Ye H, Ma D, Jiang Y, Ellen Grant P, Griswold MA, Wald LL. Improved magnetic resonance fingerprinting reconstruction with low-rank and subspace modeling. Magn Reson Med. 2018;79(2):933-942.
3. Christodoulou AG, Shaw JL, Nguyen C, Yang Q, Xie Y, Wang N, Li D. Magnetic resonance multitasking for motion-resolved quantitative cardiovascular imaging. Nat Biomed Eng 2018;2(4):215-226.
4. Wang F, Dong Z, Reese TG, Wald LL, Setsompop K. 3D-EPTI for Ultra-fast Multi-contrast and Quantitative Imaging. 2019; Montreal. p 944.
5. Wang F, Dong Z, Reese TG, Bilgic B, Katherine Manhard M, Chen J, Polimeni JR, Wald LL, Setsompop K. Echo planar time-resolved imaging (EPTI). Magn Reson Med. 2019;81(6):3599-3615.
6. Dong Z, Wang F, Reese TG, Bilgic B, Setsompop K. Echo planar time-resolved imaging with subspace reconstruction and optimized spatiotemporal encoding. Magn Reson Med. 2020.
7. Liang Z-P. Spatiotemporal imaging with partially separable functions. 2007. IEEE. p 988-991.
8. Tamir JI, Uecker M, Chen W, Lai P, Alley MT, Vasanawala SS, Lustig M. T2 shuffling: sharp, multicontrast, volumetric fast spin-echo imaging. Magn Reson Med. 2017;77(1):180-195.
Figures